Necroptosis Execution Is Mediated by Plasma Membrane Nanopores Independent of Calcium
- PMID: 28380356
- PMCID: PMC5465952
- DOI: 10.1016/j.celrep.2017.03.024
Necroptosis Execution Is Mediated by Plasma Membrane Nanopores Independent of Calcium
Abstract
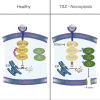
Necroptosis is a form of regulated necrosis that results in cell death and content release after plasma membrane permeabilization. However, little is known about the molecular events responsible for the disruption of the plasma membrane. Here, we find that early increase in cytosolic calcium in TNF-induced necroptosis is mediated by treatment with a Smac mimetic via the TNF/RIP1/TAK1 survival pathway. This does not require the activation of the necrosome and is dispensable for necroptosis. Necroptosis induced by the activation of TLR3/4 pathways does not trigger early calcium flux. We also demonstrate that necroptotic plasma membrane rupture is mediated by osmotic forces and membrane pores around 4 nm in diameter. This late permeabilization step represents a hallmark in necroptosis execution that is cell and treatment independent and requires the RIP1/RIP3/MLKL core. In support of this, treatment with osmoprotectants reduces cell damage in an in vivo necroptosis model of ischemia-reperfusion injury.
Keywords: Smac mimetics; TNF; calcium signaling; membrane pores; necroptosis.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

