Mitochondrial Patch Clamp of Beige Adipocytes Reveals UCP1-Positive and UCP1-Negative Cells Both Exhibiting Futile Creatine Cycling
- PMID: 28380374
- PMCID: PMC5448977
- DOI: 10.1016/j.cmet.2017.03.002
Mitochondrial Patch Clamp of Beige Adipocytes Reveals UCP1-Positive and UCP1-Negative Cells Both Exhibiting Futile Creatine Cycling
Abstract
Cold and other environmental factors induce "browning" of white fat depots-development of beige adipocytes with morphological and functional resemblance to brown fat. Similar to brown fat, beige adipocytes are assumed to express mitochondrial uncoupling protein 1 (UCP1) and are thermogenic due to the UCP1-mediated H+ leak across the inner mitochondrial membrane. However, this assumption has never been tested directly. Herein we patch clamped the inner mitochondrial membrane of beige and brown fat to provide a direct comparison of their thermogenic H+ leak (IH). All inguinal beige adipocytes had robust UCP1-dependent IH comparable to brown fat, but it was about three times less sensitive to purine nucleotide inhibition. Strikingly, only ∼15% of epididymal beige adipocytes had IH, while in the rest UCP1-dependent IH was undetectable. Despite the absence of UCP1 in the majority of epididymal beige adipocytes, these cells employ prominent creatine cycling as a UCP1-independent thermogenic mechanism.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
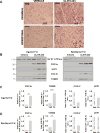


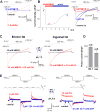
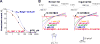

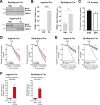
Comment in
-
Now UCP(rotein), Now You Don't: UCP1 Is Not Mandatory for Thermogenesis.Cell Metab. 2017 Apr 4;25(4):761-762. doi: 10.1016/j.cmet.2017.03.013. Cell Metab. 2017. PMID: 28380369
References
-
- Betz MJ, Enerbäck S. Human Brown Adipose Tissue: What We Have Learned So Far. Diabetes. 2015;64:2352–2360. - PubMed
-
- Borecky J, Jezek P, Siemen D. 108-pS channel in brown fat mitochondria might Be identical to the inner membrane anion channel. J Biol Chem. 1997;272:19282–19289. - PubMed
-
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

