A Class of Reactive Acyl-CoA Species Reveals the Non-enzymatic Origins of Protein Acylation
- PMID: 28380375
- PMCID: PMC5399522
- DOI: 10.1016/j.cmet.2017.03.006
A Class of Reactive Acyl-CoA Species Reveals the Non-enzymatic Origins of Protein Acylation
Abstract
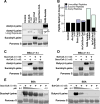
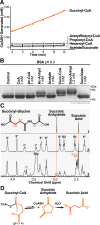
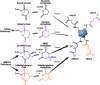
The mechanisms underlying the formation of acyl protein modifications remain poorly understood. By investigating the reactivity of endogenous acyl-CoA metabolites, we found a class of acyl-CoAs that undergo intramolecular catalysis to form reactive intermediates that non-enzymatically modify proteins. Based on this mechanism, we predicted, validated, and characterized a protein modification: 3-hydroxy-3-methylglutaryl(HMG)-lysine. In a model of altered HMG-CoA metabolism, we found evidence of two additional protein modifications: 3-methylglutaconyl(MGc)-lysine and 3-methylglutaryl(MG)-lysine. Using quantitative proteomics, we compared the "acylomes" of two reactive acyl-CoA species, namely HMG-CoA and glutaryl-CoA, which are generated in different pathways. We found proteins that are uniquely modified by each reactive metabolite, as well as common proteins and pathways. We identified the tricarboxylic acid cycle as a pathway commonly regulated by acylation and validated malate dehydrogenase as a key target. These data uncover a fundamental relationship between reactive acyl-CoA species and proteins and define a new regulatory paradigm in metabolism.
Keywords: acyl-CoA; chemical biology; non-enzymatic; post-translational modifications; protein acylation; sirtuins.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Comment in
-
Cycling around Lysine Modifications.Trends Biochem Sci. 2017 Jul;42(7):501-503. doi: 10.1016/j.tibs.2017.05.006. Epub 2017 Jun 6. Trends Biochem Sci. 2017. PMID: 28595832 Free PMC article.
References
-
- Bender ML. Mechanisms of Catalysis of Nucleophilic Reactions of Carboxylic Acid Derivatives. Chem Rev. 1960;60:53–113.
-
- Buckel W. Acetic anhydride: an intermediate analogue in the acyl-exchange reaction of citramalate lyase. Eur J Biochem. 1976;64:263–267. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

