Phase I Clinical Trial of Autologous Stem Cell-Sheet Transplantation Therapy for Treating Cardiomyopathy
- PMID: 28381469
- PMCID: PMC5532985
- DOI: 10.1161/JAHA.116.003918
Phase I Clinical Trial of Autologous Stem Cell-Sheet Transplantation Therapy for Treating Cardiomyopathy
Abstract
Background: When transplanted into failing heart, autologous somatic tissue-derived cells yield functional recovery via paracrine effects that enhance native regeneration. However, the therapeutic effects are modest. We developed a method in which scaffold-free cell sheets are attached to the epicardial surface to maximize paracrine effects. This Phase I clinical trial tested whether transplanting autologous cell-sheets derived from skeletal muscle is feasible, safe, and effective for treating severe congestive heart failure.
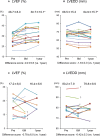
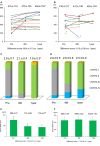
Methods and results: Fifteen ischemic cardiomyopathy patients and 12 patients with dilated cardiomyopathy, who were in New York Heart Association functional class II or III and had been treated with the maximum medical and/or interventional therapies available, were enrolled. Scaffold-free cell sheets of 3 to 9×108 cells derived from autologous muscle were transplanted over the LV free wall via left thoracotomy, without additional interventional treatments. There were no procedure-related major complications during follow-up. The majority of the ischemic cardiomyopathy patients showed marked symptomatic improvement in New York Heart Association classification (pre: 2.9±0.5 versus 6 months: 2.1±0.4, P<0.01; 1 year: 1.9±0.3, P<0.01) and the Six-Minute Walk Test with significant reduction of serum brain natriuretic peptide level (pre: 308±72 pg/mL versus 6 months: 191±56 versus 1 year: 182±46, P<0.05), pulmonary artery pressure, pulmonary capillary wedge pressure, pulmonary vein resistance, and left ventricular wall stress after transplantation instead of limited efficacy in dilated cardiomyopathy patients.
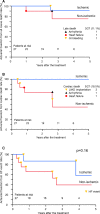
Conclusions: Cell-sheet transplantation as a sole therapy was feasible for treating cardiomyopathy. Promising results in the safety and functional recovery warrant further clinical follow-up and larger studies to confirm this treatment's efficacy for severe congestive heart failure.
Clinical trial registration: URL: http://www.umin.ac.jp/english/. Unique identifier: UMIN000003273.
Keywords: autologous stem cell‐sheet; cellular transplantation; ejection fraction; growth factors and cytokines; ischemic cardiomyopathy; myocardial regeneration.
© 2017 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures



References
-
- Miyagawa S, Roth M, Saito A, Sawa Y, Kostin S. Tissue‐engineered cardiac constructs for cardiac repair. Ann Thorac Surg. 2011;91:320–329. - PubMed
-
- Miyagawa S, Saito A, Sakaguchi T, Yoshikawa Y, Yamauchi T, Imanishi Y. Impaired myocardium regeneration with skeletal cell sheets–a preclinical trial for tissue‐engineered regeneration therapy. Transplantation. 2010;90:364–372. - PubMed
-
- Memon IA, Sawa Y, Fukushima N, Matsumiya G, Miyagawa S, Taketani S, Sakakida S, Kondoh H, Aleshin AN, Shimizu T, Okano T, Matsuda H. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J Thorac Cardiovasc Surg. 2005;130:1333–1341. - PubMed
-
- Menasché P, Vanneaux V, Hagège A, Bel A, Cholley B, Cacciapuoti I, Parouchev A, Benhamouda N, Tachdjian G, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Guillemain R, Suberbielle Boissel C, Tartour E, Desnos M, Larghero J. Human embryonic stem cell‐derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J. 2015;36:2011–2017. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

