Neural coding of time-varying interaural time differences and time-varying amplitude in the inferior colliculus
- PMID: 28381487
- PMCID: PMC5511866
- DOI: 10.1152/jn.00797.2016
Neural coding of time-varying interaural time differences and time-varying amplitude in the inferior colliculus
Abstract
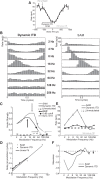
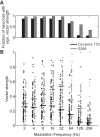
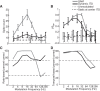
Binaural cues occurring in natural environments are frequently time varying, either from the motion of a sound source or through interactions between the cues produced by multiple sources. Yet, a broad understanding of how the auditory system processes dynamic binaural cues is still lacking. In the current study, we directly compared neural responses in the inferior colliculus (IC) of unanesthetized rabbits to broadband noise with time-varying interaural time differences (ITD) with responses to noise with sinusoidal amplitude modulation (SAM) over a wide range of modulation frequencies. On the basis of prior research, we hypothesized that the IC, one of the first stages to exhibit tuning of firing rate to modulation frequency, might use a common mechanism to encode time-varying information in general. Instead, we found weaker temporal coding for dynamic ITD compared with amplitude modulation and stronger effects of adaptation for amplitude modulation. The differences in temporal coding of dynamic ITD compared with SAM at the single-neuron level could be a neural correlate of "binaural sluggishness," the inability to perceive fluctuations in time-varying binaural cues at high modulation frequencies, for which a physiological explanation has so far remained elusive. At ITD-variation frequencies of 64 Hz and above, where a temporal code was less effective, noise with a dynamic ITD could still be distinguished from noise with a constant ITD through differences in average firing rate in many neurons, suggesting a frequency-dependent tradeoff between rate and temporal coding of time-varying binaural information.NEW & NOTEWORTHY Humans use time-varying binaural cues to parse auditory scenes comprising multiple sound sources and reverberation. However, the neural mechanisms for doing so are poorly understood. Our results demonstrate a potential neural correlate for the reduced detectability of fluctuations in time-varying binaural information at high speeds, as occurs in reverberation. The results also suggest that the neural mechanisms for processing time-varying binaural and monaural cues are largely distinct.
Keywords: amplitude modulation; auditory motion; binaural sluggishness; inferior colliculus; reverberation.
Copyright © 2017 the American Physiological Society.
Figures












References
-
- Bernstein LR. Detection and discrimination of interaural disparities: modern earphone-based studies. In: Binaural and Spatial Hearing in Real and Virtual Environments, edited by Gilkey R and Anderson TR. Mahwah, NJ: Erlbaum, 1997, p. 117–138.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

