Parallel processing of internal and external feedback in the spinocerebellar system of primates
- PMID: 28381489
- PMCID: PMC5498734
- DOI: 10.1152/jn.00825.2016
Parallel processing of internal and external feedback in the spinocerebellar system of primates
Abstract
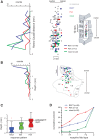
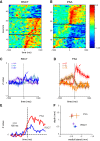
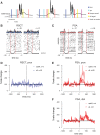
Cerebellar control of voluntary movements is achieved by the integration of external and internal feedback information to adjust and correct properly ongoing actions. In the forelimb of primates, rostral-spinocerebellar tract (RSCT) neurons are thought to integrate segmental, descending, and afferent sources and relay upstream a compound signal that contains both an efference copy of the spinal-level motor command and the state of the periphery. We tested this hypothesis by implanting stimulating electrodes in the superior cerebellar peduncle and recording the activity of cervical spinal neurons in primates. To dissociate motor commands and proprioceptive signals, we used a voluntary wrist task and applied external perturbations to the movement. We identified a large group of antidromically activated RSCT neurons located in deep dorsal sites and a smaller fraction of postsynaptically activated (PSA) cells located in intermediate and ventral laminae. RSCT cells received sensory input from broad, proximally biased receptive fields (RFs) and were not affected by applied wrist perturbations. PSA cells received sensory information from distal RFs and were more strongly related to active and passive movements. The anatomical and functional properties of RSCT and PSA cells suggest that descending signals converging on PSA cells contribute to both motor preparation and motor control. In parallel, RSCT neurons relay upstream an integrated signal that encodes the state of working muscles and can contribute to distal-to-proximal coordination of action. Thus the rostral spinocerebellar system sends upstream an efference copy of the motor command but does not signal abrupt errors in the performed movement.NEW & NOTEWORTHY Cerebellar coordination of voluntary movements relies on integrating feedback information to update motor output. With the use of a novel protocol, we identified spinal neurons constituting the ascending and descending components of the forelimb spinocerebellar system in behaving primates. The data suggest that descending information contributes to both motor preparation and execution, whereas ascending information conveys the spinal level motor command, such that internal and external feedback is relayed through parallel pathways.
Keywords: motor control; primates; spinocerebellar; voluntary movements.
Copyright © 2017 the American Physiological Society.
Figures




Similar articles
-
Structure of Long-Range Direct and Indirect Spinocerebellar Pathways as Well as Local Spinal Circuits Mediating Proprioception.J Neurosci. 2022 Jan 26;42(4):581-600. doi: 10.1523/JNEUROSCI.2157-20.2021. Epub 2021 Dec 2. J Neurosci. 2022. PMID: 34857649 Free PMC article.
-
Information to cerebellum on spinal motor networks mediated by the dorsal spinocerebellar tract.J Physiol. 2013 Nov 15;591(22):5433-43. doi: 10.1113/jphysiol.2012.249110. Epub 2013 Apr 22. J Physiol. 2013. PMID: 23613538 Free PMC article. Review.
-
Rhythmic activity of feline dorsal and ventral spinocerebellar tract neurons during fictive motor actions.J Neurophysiol. 2013 Jan;109(2):375-88. doi: 10.1152/jn.00649.2012. Epub 2012 Oct 24. J Neurophysiol. 2013. PMID: 23100134
-
The lateral reticular nucleus; integration of descending and ascending systems regulating voluntary forelimb movements.Front Comput Neurosci. 2015 Aug 5;9:102. doi: 10.3389/fncom.2015.00102. eCollection 2015. Front Comput Neurosci. 2015. PMID: 26300768 Free PMC article.
-
Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus.Physiol Rev. 1986 Jan;66(1):118-71. doi: 10.1152/physrev.1986.66.1.118. Physiol Rev. 1986. PMID: 3511480 Review.
Cited by
-
Local-to-distant development of the cerebrocerebellar sensorimotor network in the typically developing human brain: a functional and diffusion MRI study.Brain Struct Funct. 2019 Apr;224(3):1359-1375. doi: 10.1007/s00429-018-01821-5. Epub 2019 Feb 7. Brain Struct Funct. 2019. PMID: 30729998 Free PMC article.
-
Disentangling acute motor deficits and adaptive responses evoked by the loss of cerebellar output.Elife. 2025 Jun 25;14:RP105152. doi: 10.7554/eLife.105152. Elife. 2025. PMID: 40560643 Free PMC article.
-
Sensorimotor anatomy of gait, balance, and falls.Handb Clin Neurol. 2018;159:3-26. doi: 10.1016/B978-0-444-63916-5.00001-X. Handb Clin Neurol. 2018. PMID: 30482322 Free PMC article. Review.
-
Normal-Appearing Cerebellar Damage in Neuromyelitis Optica Spectrum Disorder.AJNR Am J Neuroradiol. 2019 Jul;40(7):1156-1161. doi: 10.3174/ajnr.A6098. Epub 2019 Jun 20. AJNR Am J Neuroradiol. 2019. PMID: 31221630 Free PMC article.
-
Control of Mammalian Locomotion by Somatosensory Feedback.Compr Physiol. 2021 Dec 29;12(1):2877-2947. doi: 10.1002/cphy.c210020. Compr Physiol. 2021. PMID: 34964114 Free PMC article.
References
-
- Alstermark B, Lindström S, Lundberg A, Sybirska E. Integration in descending motor pathways controlling the forelimb in the cat. 8. Ascending projection to the lateral reticular nucleus from C3-C4 propriospinal also projecting to forelimb motoneurones. Exp Brain Res 42: 282–298, 1981. doi:10.1007/BF00237495. - DOI - PubMed
-
- Belhaj-Saïf A, Karrer JH, Cheney PD. Distribution and characteristics of poststimulus effects in proximal and distal forelimb muscles from red nucleus in the monkey. J Neurophysiol 79: 1777–1789, 1998. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

