PECTIN METHYLESTERASE34 Contributes to Heat Tolerance through Its Role in Promoting Stomatal Movement
- PMID: 28381503
- PMCID: PMC5462046
- DOI: 10.1104/pp.17.00335
PECTIN METHYLESTERASE34 Contributes to Heat Tolerance through Its Role in Promoting Stomatal Movement
Abstract
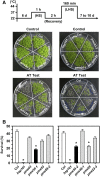
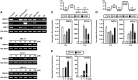
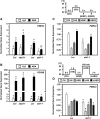
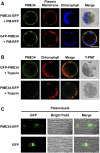
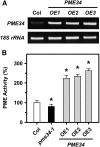
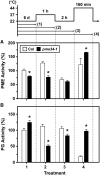
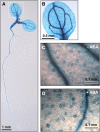
Pectin, a major component of the primary cell wall, is synthesized in the Golgi apparatus and exported to the cell wall in a highly methylesterified form, then is partially demethylesterified by pectin methylesterases (PMEs; EC 3.1.1.11). PME activity on the status of pectin methylesterification profoundly affects the properties of pectin and, thereby, is critical for plant development and the plant defense response, although the roles of PMEs under heat stress (HS) are poorly understood. Functional genome annotation predicts that at least 66 potential PME genes are contained in Arabidopsis (Arabidopsis thaliana). Thermotolerance assays of PME gene T-DNA insertion lines revealed two null mutant alleles of PME34 (At3g49220) that both consistently showed reduced thermotolerance. Nevertheless, their impairment was independently associated with the expression of HS-responsive genes. It was also observed that PME34 transcription was induced by abscisic acid and highly expressed in guard cells. We showed that the PME34 mutation has a defect in the control of stomatal movement and greatly altered PME and polygalacturonase (EC 3.2.1.15) activity, resulting in a heat-sensitive phenotype. PME34 has a role in the regulation of transpiration through the control of the stomatal aperture due to its cell wall-modifying enzyme activity during the HS response. Hence, PME34 is required for regulating guard cell wall flexibility to mediate the heat response in Arabidopsis.
© 2017 American Society of Plant Biologists. All Rights Reserved.
Figures





References
-
- Alexandrov VY (1994) Functional aspects of cell response to heat shock. Int Rev Cytol 148: 171–227 - PubMed
-
- Baron KN, Schroeder DF, Stasolla C (2012) Transcriptional response of abscisic acid (ABA) metabolism and transport to cold and heat stress applied at the reproductive stage of development in Arabidopsis thaliana. Plant Sci 188-189: 48–59 - PubMed
-
- Bellincampi D, Camardella L, Delcour JA, Desseaux V, D’Ovidio R, Durand A, Elliot G, Gebruers K, Giovane A, Juge N, et al. (2004) Potential physiological role of plant glycosidase inhibitors. Biochim Biophys Acta 1696: 265–274 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

