Production of homogeneous glycoprotein with multisite modifications by an engineered N-glycosyltransferase mutant
- PMID: 28381551
- PMCID: PMC5448120
- DOI: 10.1074/jbc.M117.777383
Production of homogeneous glycoprotein with multisite modifications by an engineered N-glycosyltransferase mutant
Abstract
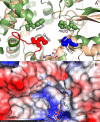
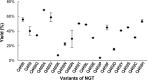
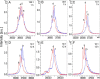
Naturally occurring N-glycoproteins exhibit glycoform heterogeneity with respect to N-glycan sequon occupancy (macroheterogeneity) and glycan structure (microheterogeneity). However, access to well-defined glycoproteins is always important for both basic research and therapeutic purposes. As a result, there has been a substantial effort to identify and understand the catalytic properties of N-glycosyltransferases, enzymes that install the first glycan on the protein chain. In this study we found that ApNGT, a newly discovered cytoplasmic N-glycosyltransferase from Actinobacillus pleuropneumoniae, has strict selectivity toward the residues around the Asn of N-glycosylation sequon by screening a small library of synthetic peptides. The inherent stringency was subsequently demonstrated to be closely associated with a critical residue (Gln-469) of ApNGT which we propose hinders the access of bulky residues surrounding the occupied Asn into the active site. Site-saturated mutagenesis revealed that the introduction of small hydrophobic residues at the site cannot only weaken the stringency of ApNGT but can also contribute to enormous improvement of glycosylation efficiency against both short peptides and proteins. We then employed the most efficient mutant (Q469A) other than the wild-type ApNGT to produce a homogeneous glycoprotein carrying multiple (up to 10) N-glycans, demonstrating that this construct is a promising biocatalyst for potentially addressing the issue of macroheterogeneity in glycoprotein preparation.
Keywords: N-linked glycosylation; glycoprotein; glycosyltransferase; post-translational modification (PTM); protein engineering.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

