Early B Cell Progenitors Deficient for GON4L Fail To Differentiate Due to a Block in Mitotic Cell Division
- PMID: 28381640
- PMCID: PMC5444326
- DOI: 10.4049/jimmunol.1602054
Early B Cell Progenitors Deficient for GON4L Fail To Differentiate Due to a Block in Mitotic Cell Division
Abstract
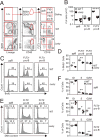
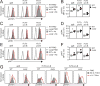
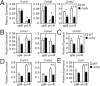
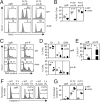
B cell development in Justy mutant mice is blocked due to a precursor mRNA splicing defect that depletes the protein GON4-like (GON4L) in B cell progenitors. Genetic and biochemical studies have suggested that GON4L is a transcriptional regulator that coordinates cell division with differentiation, but its role in B cell development is unknown. To understand the function of GON4L, we characterized B cell differentiation, cell cycle control, and mitotic gene expression in GON4L-deficient B cell progenitors from Justy mice. We found that these cells established key aspects of the transcription factor network that guides B cell development and proliferation and rearranged the IgH gene locus. However, despite intact IL-7 signaling, GON4L-deficient pro-B cell stage precursors failed to undergo a characteristic IL-7-dependent proliferative burst. These cells also failed to upregulate genes required for mitotic division, including those encoding the G1/S cyclin D3 and E2F transcription factors and their targets. Additionally, GON4L-deficient B cell progenitors displayed defects in DNA synthesis and passage through the G1/S transition, contained fragmented DNA, and underwent apoptosis. These phenotypes were not suppressed by transgenic expression of prosurvival factors. However, transgenic expression of cyclin D3 or other regulators of the G1/S transition restored pro-B cell development from Justy progenitor cells, suggesting that GON4L acts at the beginning of the cell cycle. Together, our findings indicate that GON4L is essential for cell cycle progression and division during the early stages of B cell development.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures


Similar articles
-
The transcriptional regulator GON4L is required for viability and hematopoiesis in mice.Exp Hematol. 2021 Jun;98:25-35. doi: 10.1016/j.exphem.2021.04.001. Epub 2021 Apr 14. Exp Hematol. 2021. PMID: 33864850 Free PMC article.
-
The Justy mutation identifies Gon4-like as a gene that is essential for B lymphopoiesis.J Exp Med. 2010 Jul 5;207(7):1359-67. doi: 10.1084/jem.20100147. Epub 2010 Jun 7. J Exp Med. 2010. PMID: 20530203 Free PMC article.
-
Developmental stage-specific effects of Pim-1 dysregulation on murine bone marrow B cell development.BMC Immunol. 2016 Jun 10;17(1):16. doi: 10.1186/s12865-016-0152-1. BMC Immunol. 2016. PMID: 27287229 Free PMC article.
-
Fidelity and infidelity in commitment to B-lymphocyte lineage development.Immunol Rev. 2000 Jun;175:104-11. Immunol Rev. 2000. PMID: 10933595 Review.
-
Regulation of the G1/S transition phase in mesangial cells by E2F1.Kidney Int. 1999 Oct;56(4):1238-41. doi: 10.1046/j.1523-1755.1999.00705.x. Kidney Int. 1999. PMID: 10504464 Review.
Cited by
-
SVFX: a machine learning framework to quantify the pathogenicity of structural variants.Genome Biol. 2020 Nov 9;21(1):274. doi: 10.1186/s13059-020-02178-x. Genome Biol. 2020. PMID: 33168059 Free PMC article.
-
Newly identified Gon4l/Udu-interacting proteins implicate novel functions.Sci Rep. 2020 Aug 26;10(1):14213. doi: 10.1038/s41598-020-70855-9. Sci Rep. 2020. PMID: 32848183 Free PMC article.
-
Biallelic loss-of-function variants in GON4L cause microcephaly and brain structure abnormalities.NPJ Genom Med. 2024 Nov 5;9(1):55. doi: 10.1038/s41525-024-00437-5. NPJ Genom Med. 2024. PMID: 39500882 Free PMC article.
-
The transcriptional regulator GON4L is required for viability and hematopoiesis in mice.Exp Hematol. 2021 Jun;98:25-35. doi: 10.1016/j.exphem.2021.04.001. Epub 2021 Apr 14. Exp Hematol. 2021. PMID: 33864850 Free PMC article.
-
Structural Analysis of the SANT/Myb Domain of FLASH and YARP Proteins and Their Complex with the C-Terminal Fragment of NPAT by NMR Spectroscopy and Computer Simulations.Int J Mol Sci. 2020 Jul 24;21(15):5268. doi: 10.3390/ijms21155268. Int J Mol Sci. 2020. PMID: 32722282 Free PMC article.
References
-
- Nutt SL, Kee BL. The transcriptional regulation of B cell lineage commitment. Immunity. 2007;26:715–725. - PubMed
-
- Mandel EM, Grosschedl R. Transcription control of early B cell differentiation. Curr Opin Immunol. 2010;22:161–167. - PubMed
-
- Reth M, Nielsen P. Signaling circuits in early B-cell development. Adv Immunol. 2014;122:129–175. - PubMed
-
- Bain G, Maandag EC, Izon DJ, Amsen D, Kruisbeek AM, Weintraub BC, Krop I, Schlissel MS, Feeney AJ, van Roon M, van der Valk M, Riele HPJ, Berns A, Murre C. E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements. Cell. 1994;79:885–892. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

