Recent Advances in Fluorescent Angioscopy for Molecular Imaging of Human Atherosclerotic Coronary Plaque
- PMID: 28381766
- PMCID: PMC5453678
- DOI: 10.5551/jat.40352
Recent Advances in Fluorescent Angioscopy for Molecular Imaging of Human Atherosclerotic Coronary Plaque
Abstract
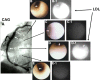
Purpose of review: In vivo imaging of the native substances, including lipoproteins, that comprise human atherosclerotic plaques is currently beyond the scope of any available imaging techniques. Color and near-infrared fluorescent angioscopy (CFA and NIRFA, respectively) systems have been recently developed for molecular imaging of lipoproteins within the human coronary arterial wall ex vivo and/or in vivo. The author reviews recent findings on lipoprotein deposition in human coronary plaques obtained by these imaging techniques.
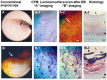
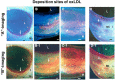
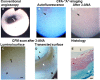
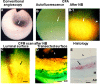
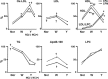
Recent findings: Using specific biomarkers, native pro-atherogenic substances such as oxidized low-density lipoprotein (ox-LDL), LDL, triglycerides (TG), apolipoprotein B-100 (ApoB-100), and lysophosphatidylcholine (LPC), and the anti-atherogenic substance such as high-density lipoprotein (HDL) were visualized by CFA, and LDL and cholesterol by NIRFA, in coronary plaques obtained from autopsy subjects. The relationship between incidence and plaque morphology differed for each substance. The incidence of ox-LDL and LDL on color fluorescence microscopy correlated well with that observed using immunohistochemical techniques. During coronary catheterization in patients, ox-LDL, LDL, and HDL in coronary plaques were visualized by CFA or NIRFA.
Conclusions: Using CFA or NIRFA, the distribution of the major native pro-atherogenic and anti-atherogenic lipoproteins and their components within human coronary plaques can be evaluated ex vivo and/or in vivo. Fluorescent angioscopy could help our understanding of the molecular mechanisms of coronary atherosclerosis and in the evaluation of the effects of therapy targeting the substances comprising atherosclerotic coronary plaques.
Keywords: Apolipoproteins; Biomarkers; Color fluorescent angioscopy; Immunohistochemistry; Lipoproteins; Near-infrared fluorescent angioscopy.
Figures





References
-
- Matsuura E, Hughes GR, Khamashta MA: Oxidation of LDL and its clinical implication. Autoimmun Rev, 2008; 7: 558-566 - PubMed
-
- Chen JH, Riazzy, Smith EM, Proud CG, Steinbrecher U: Oxidized LDL-mediated macrophage survival involves elongation of factor-2 kinase. Arterioscler Thromb Vasc Biol, 2009; 29: 92-98 - PubMed
-
- Tardif JC: Emerging high-density lipoprotein infusion therapies: fulfilling the promise of epidemiology? J Clin Lipidol, 2010; 4: 399-404 - PubMed
-
- Negi S, Ballantyne CM: Insights from recent meta-analysis: role of high-density lipoprotein cholesterol in reducing cardiovascular events and rates of atherosclerotic disease progression. J Clin Lipidol, 2010; 4: 365-370 - PubMed
-
- Vilani SS, Nambi V: The role of lipoprotein-associated phospholipse A2 as a marker of atherosclerosis. Curr Atheroscler Rep, 2007; 9: 97-103 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

