Neutrophil Extracellular Traps of Cynoglossus semilaevis: Production Characteristics and Antibacterial Effect
- PMID: 28382034
- PMCID: PMC5360709
- DOI: 10.3389/fimmu.2017.00290
Neutrophil Extracellular Traps of Cynoglossus semilaevis: Production Characteristics and Antibacterial Effect
Abstract
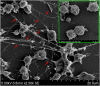
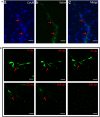
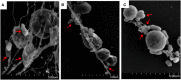
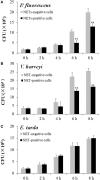
Neutrophil extracellular traps (NETs) are structures released by neutrophils as a cellular immune defense against microbial invasion. The process of NETs generation, netosis (NETosis), can take place via either a suicidal mechanism, during which the NETs-releasing cells became dead, or a "live" mechanism, during which the NETs-releasing cells remain vital. NETosis has been studied intensively in mammals in recent years, but very little is known about the NETosis in fish. In this study, we examined NETosis in tongue sole (Cynoglossus semilaevis), a species of teleost with important economic values. We found that following stimulation with phorbol 12-myristate 13-acetate (PMA) and three common fish bacterial pathogens, abundant NETs structures were released by neutrophils that were most likely in a live state. The released NETs captured, but did not kill, the bacterial pathogens; however, the replication of extracellular, but not intracellular, pathogens was inhibited by NETs to significant extents. Reactive oxygen species (ROS), nitric oxide (NO), and myeloperoxidase (MPO) production were observed to be enhanced in NETosing neutrophils, and blocking the production of these factors by inhibitors significantly decreased NETs production induced by PMA and all three bacteria. Taken together, these results indicate for the first time that in teleost there exists a non-cell death pathway of NETosis that produces NETs with antibacterial effects in a ROS-, NO-, and MPO-dependent manner.
Keywords: Cynoglossus semilaevis; NETosis; antibacterial; innate immune defense; neutrophil extracellular trap.
Figures


Similar articles
-
Peptidylarginine Deiminase Inhibitor Suppresses Neutrophil Extracellular Trap Formation and MPO-ANCA Production.Front Immunol. 2016 Jun 8;7:227. doi: 10.3389/fimmu.2016.00227. eCollection 2016. Front Immunol. 2016. PMID: 27375623 Free PMC article.
-
Dexamethasone Inhibits S. aureus-Induced Neutrophil Extracellular Pathogen-Killing Mechanism, Possibly through Toll-Like Receptor Regulation.Front Immunol. 2017 Feb 9;8:60. doi: 10.3389/fimmu.2017.00060. eCollection 2017. Front Immunol. 2017. PMID: 28232829 Free PMC article.
-
(+)-Borneol inhibits the generation of reactive oxygen species and neutrophil extracellular traps induced by phorbol-12-myristate-13-acetate.Front Pharmacol. 2022 Nov 7;13:1023450. doi: 10.3389/fphar.2022.1023450. eCollection 2022. Front Pharmacol. 2022. PMID: 36419617 Free PMC article.
-
[Progress in mechanism of formation of neutrophil extracellular traps: Review].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2020 Jun;36(6):561-564. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2020. PMID: 32696748 Review. Chinese.
-
Neutrophil Extracellular Traps in Periodontitis: A Web of Intrigue.J Dent Res. 2016 Jan;95(1):26-34. doi: 10.1177/0022034515609097. Epub 2015 Oct 6. J Dent Res. 2016. PMID: 26442948 Review.
Cited by
-
Pseudomonas plecoglossicida infection induces neutrophil autophagy-driven NETosis in large yellow croaker Larimichthys crocea.Front Immunol. 2024 Dec 23;15:1521080. doi: 10.3389/fimmu.2024.1521080. eCollection 2024. Front Immunol. 2024. PMID: 39763642 Free PMC article.
-
Neutrophils and aquatic pathogens.Parasite Immunol. 2022 Jun;44(6):e12915. doi: 10.1111/pim.12915. Epub 2022 Mar 22. Parasite Immunol. 2022. PMID: 35290688 Free PMC article. Review.
-
Extracellular DNA traps in a ctenophore demonstrate immune cell behaviors in a non-bilaterian.Nat Commun. 2024 Apr 6;15(1):2990. doi: 10.1038/s41467-024-46807-6. Nat Commun. 2024. PMID: 38582801 Free PMC article.
-
Chlorpyrifos Suppresses Neutrophil Extracellular Traps in Carp by Promoting Necroptosis and Inhibiting Respiratory Burst Caused by the PKC/MAPK Pathway.Oxid Med Cell Longev. 2019 Feb 7;2019:1763589. doi: 10.1155/2019/1763589. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 30881588 Free PMC article.
-
NETosis in Rheumatic Diseases.Curr Rheumatol Rep. 2021 Jan 28;23(2):9. doi: 10.1007/s11926-020-00977-6. Curr Rheumatol Rep. 2021. PMID: 33511473 Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

