Theranostic DNAzymes
- PMID: 28382172
- PMCID: PMC5381262
- DOI: 10.7150/thno.17736
Theranostic DNAzymes
Abstract
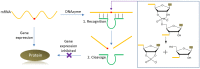
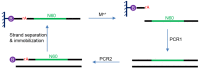
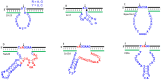
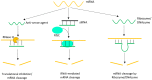
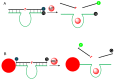
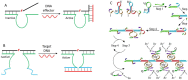
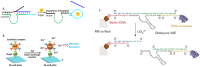
DNAzymes are catalytically active DNA molecules that are obtained via in vitro selection. RNA-cleaving DNAzymes have attracted significant attention for both therapeutic and diagnostic applications due to their excellent programmability, stability, and activity. They can be designed to cleave a specific mRNA to down-regulate gene expression. At the same time, DNAzymes can sense a broad range of analytes. By combining these two functions, theranostic DNAzymes are obtained. This review summarizes the progress of DNAzyme for theranostic applications. First, in vitro selection of DNAzymes is briefly introduced, and some representative DNAzymes related to biological applications are summarized. Then, the applications of DNAzyme for RNA cleaving are reviewed. DNAzymes have been used to cleave RNA for treating various diseases, such as viral infection, cancer, inflammation and atherosclerosis. Several formulations have entered clinical trials. Next, the use of DNAzymes for detecting metal ions, small molecules and nucleic acids related to disease diagnosis is summarized. Finally, the theranostic applications of DNAzyme are reviewed. The challenges to be addressed include poor DNAzyme activity under biological conditions, mRNA accessibility, delivery, and quantification of gene expression. Possible solutions to overcome these challenges are discussed, and future directions of the field are speculated.
Keywords: DNAzymes; RNA; biosensors; delivery.; metal ions.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
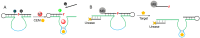
Similar articles
-
Discovery and Biosensing Applications of Diverse RNA-Cleaving DNAzymes.Acc Chem Res. 2017 Sep 19;50(9):2273-2283. doi: 10.1021/acs.accounts.7b00262. Epub 2017 Aug 14. Acc Chem Res. 2017. PMID: 28805376
-
Zn2+ -Dependent DNAzymes: From Solution Chemistry to Analytical, Materials and Therapeutic Applications.Chembiochem. 2021 Mar 2;22(5):779-789. doi: 10.1002/cbic.202000586. Epub 2020 Nov 11. Chembiochem. 2021. PMID: 33007113 Review.
-
Sensitive dual DNAzymes-based sensors designed by grafting self-blocked G-quadruplex DNAzymes to the substrates of metal ion-triggered DNA/RNA-cleaving DNAzymes.Biosens Bioelectron. 2012 Oct-Dec;38(1):331-6. doi: 10.1016/j.bios.2012.06.011. Epub 2012 Jun 19. Biosens Bioelectron. 2012. PMID: 22784499
-
A complex RNA-cleaving DNAzyme that can efficiently cleave a pyrimidine-pyrimidine junction.J Mol Biol. 2010 Jul 23;400(4):689-701. doi: 10.1016/j.jmb.2010.05.047. Epub 2010 May 26. J Mol Biol. 2010. PMID: 20630470
-
RNA-cleaving DNAzymes for accurate biosensing and gene therapy.Nanoscale. 2023 Jul 13;15(27):11346-11365. doi: 10.1039/d3nr01482g. Nanoscale. 2023. PMID: 37376885 Review.
Cited by
-
Bcl-xL DNAzymes promote radiosensitivity and chemosensitivity in colorectal cancer cells via enhancing apoptosis.BMC Pharmacol Toxicol. 2022 Feb 5;23(1):13. doi: 10.1186/s40360-022-00553-x. BMC Pharmacol Toxicol. 2022. PMID: 35123593 Free PMC article.
-
Development of Novel antimiRzymes for Targeted Inhibition of miR-21 Expression in Solid Cancer Cells.Molecules. 2019 Jul 7;24(13):2489. doi: 10.3390/molecules24132489. Molecules. 2019. PMID: 31284665 Free PMC article.
-
Natural polyphenol assisted delivery of single-strand oligonucleotides by cationic polymers.Gene Ther. 2020 Aug;27(7-8):383-391. doi: 10.1038/s41434-020-0151-y. Epub 2020 May 4. Gene Ther. 2020. PMID: 32366887 Free PMC article.
-
Nucleic acid-based therapy for coronavirus disease 2019.Heliyon. 2020 Sep;6(9):e05007. doi: 10.1016/j.heliyon.2020.e05007. Epub 2020 Sep 19. Heliyon. 2020. PMID: 32984620 Free PMC article. Review.
-
Clinical Perspectives of Theranostics.Molecules. 2021 Apr 13;26(8):2232. doi: 10.3390/molecules26082232. Molecules. 2021. PMID: 33924345 Free PMC article. Review.
References
-
- Kelkar SS, Reineke TM. Theranostics: combining imaging and therapy. Bioconj Chem. 2011;22:1879–903. - PubMed
-
- Lammers T, Aime S, Hennink WE, Storm G, Kiessling F. Theranostic nanomedicine. Acc Chem Res. 2011;44:1029–38. - PubMed
-
- Wang X, Hu Y, Wei H. Nanozymes in bionanotechnology: from sensing to therapeutics and beyond. Inorg Chem Front. 2016;3:41–60.
-
- Wei H, Wang E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev. 2013;42:6060–93. - PubMed
-
- Lin Y, Ren J, Qu X. Catalytically active nanomaterials: a promising candidate for artificial enzymes. Acc Chem Res. 2014;47:1097–105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

