Cysteine transporter SLC3A1 promotes breast cancer tumorigenesis
- PMID: 28382174
- PMCID: PMC5381264
- DOI: 10.7150/thno.18005
Cysteine transporter SLC3A1 promotes breast cancer tumorigenesis
Abstract
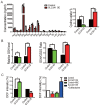
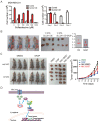
Cysteine is an essential amino acid for infants, aged people as well as patients with metabolic disorders. Although the thiol group of cysteine side chain is active in oxidative reactions, the role of cysteine in cancer remains largely unknown. Here, we report that the expression level of the solute carrier family 3, member 1 (SLC3A1), the cysteine carrier, tightly correlated with clinical stages and patients' survival. Elevated SLC3A1 expression accelerated the cysteine uptake and the accumulation of reductive glutathione (GSH), leading to reduced reactive oxygen species (ROS). ROS increased the stability and activity of PP2Ac, resulting in decreased AKT activity. Hence, SLC3A1 activated the AKT signaling through inhibiting PP2A phosphatase activity. Consistently, overexpression of SLC3A1 enhanced tumorigenesis of breast cancer cells, whereas blocking SLC3A1 either with specific siRNA or SLC3A1 specific inhibitor sulfasalazine suppressed tumor growth and also abolished dietary NAC-promoted tumor growth. Collectively, our data demonstrate that SLC3A1 promotes cysteine uptake and determines cellular response to antioxidant N-acetylcysteine, suggesting SLC3A1 is a potential therapeutic target for breast cancer.
Keywords: PDK1; ROS; breast cancer; cysteine; solute carrier SLC3A1.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
Tissue distribution, hormonal regulation, ontogeny, diurnal expression, and induction of mouse cystine transporters Slc3a1 and Slc7a9.Free Radic Res. 2020 Jul;54(7):525-534. doi: 10.1080/10715762.2020.1812597. Epub 2020 Sep 1. Free Radic Res. 2020. PMID: 32873097 Free PMC article.
-
Feline cystinuria caused by a missense mutation in the SLC3A1 gene.J Vet Intern Med. 2015 Jan;29(1):120-5. doi: 10.1111/jvim.12501. Epub 2014 Nov 24. J Vet Intern Med. 2015. PMID: 25417848 Free PMC article.
-
Digenic Inheritance in Cystinuria Mouse Model.PLoS One. 2015 Sep 11;10(9):e0137277. doi: 10.1371/journal.pone.0137277. eCollection 2015. PLoS One. 2015. PMID: 26359869 Free PMC article.
-
[From gene to disease; SLC3A1, SLC7A9 and cystinuria].Ned Tijdschr Geneeskd. 2003 Feb 8;147(6):245-7. Ned Tijdschr Geneeskd. 2003. PMID: 12621979 Review. Dutch.
-
The molecular basis of cystinuria.Nephron Exp Nephrol. 2004;98(2):e45-9. doi: 10.1159/000080255. Nephron Exp Nephrol. 2004. PMID: 15499206 Review.
Cited by
-
Inducing ferroptosis has the potential to overcome therapy resistance in breast cancer.Front Immunol. 2022 Nov 24;13:1038225. doi: 10.3389/fimmu.2022.1038225. eCollection 2022. Front Immunol. 2022. PMID: 36505465 Free PMC article. Review.
-
Identification of Malignant Cell Populations Associated with Poor Prognosis in High-Grade Serous Ovarian Cancer Using Single-Cell RNA Sequencing.Cancers (Basel). 2022 Jul 22;14(15):3580. doi: 10.3390/cancers14153580. Cancers (Basel). 2022. PMID: 35892844 Free PMC article.
-
Amino Acid Transporters and Glutamine Metabolism in Breast Cancer.Int J Mol Sci. 2018 Mar 19;19(3):907. doi: 10.3390/ijms19030907. Int J Mol Sci. 2018. PMID: 29562706 Free PMC article. Review.
-
Small Maf functions in the maintenance of germline stem cells in the Drosophila testis.Redox Biol. 2018 May;15:125-134. doi: 10.1016/j.redox.2017.12.002. Epub 2017 Dec 8. Redox Biol. 2018. PMID: 29245136 Free PMC article.
-
A Review on the Role of Human Solute Carriers Transporters in Cancer.Health Sci Rep. 2025 Jan 12;8(1):e70343. doi: 10.1002/hsr2.70343. eCollection 2025 Jan. Health Sci Rep. 2025. PMID: 39807482 Free PMC article.
References
-
- Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–91. - PubMed
-
- Ranjan P, Anathy V, Burch PM. et al. Redox-dependent expression of cyclin D1 and cell proliferation by Nox1 in mouse lung epithelial cells. Antioxid Redox Signal. 2006;8:1447–59. - PubMed
-
- Xu D, Rovira II, Finkel T. Oxidants painting the cysteine chapel: redox regulation of PTPs. Dev Cell. 2002;2:251–2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

