Structure-efficiency relationships of cyclodextrin scavengers in the hydrolytic degradation of organophosphorus compounds
- PMID: 28382180
- PMCID: PMC5355938
- DOI: 10.3762/bjoc.13.45
Structure-efficiency relationships of cyclodextrin scavengers in the hydrolytic degradation of organophosphorus compounds
Abstract
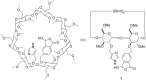
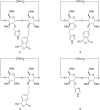
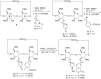
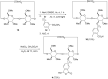
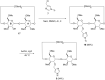
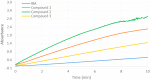
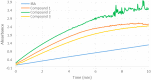
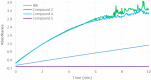
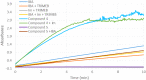
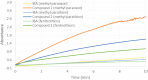
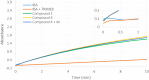
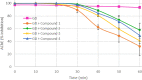
New derivatives of cyclodextrins were prepared in order to determine the relative importance of the structural key elements involved in the degradation of organophosphorus nerve agents. To avoid a competitive inclusion between the organophosphorus substrate and the iodosobenzoate group, responsible for its degradation, the latter group had to be covalently bound to the cyclodextrin scaffold. Although the presence of the α nucleophile iodosobenzoate was a determinant in the hydrolysis process, an imidazole group was added to get a synergistic effect towards the degradation of the agents. The degradation efficiency was found to be dependent on the relative position of the heterocycle towards the reactive group as well as on the nature of the organophosphorus derivative.
Keywords: cyclodextrin; decontamination; enzyme mimic; nerve agents; organophosphorus pesticides.
Figures
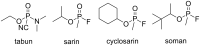
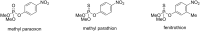
References
-
- Benschop H P, De Jong L P A. Acc Chem Res. 1988;21:368–374. doi: 10.1021/ar00154a003. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
