Paradoxical Role of High Mobility Group Box 1 in Glioma: A Suppressor or a Promoter?
- PMID: 28382190
- PMCID: PMC5364998
- DOI: 10.4081/oncol.2017.325
Paradoxical Role of High Mobility Group Box 1 in Glioma: A Suppressor or a Promoter?
Abstract
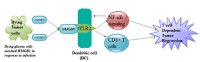
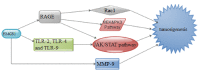
Gliomas represent 60% of primary intracranial brain tumors and 80% of all malignant types, with highest morbidity and mortality worldwide. Although glioma has been extensively studied, the molecular mechanisms underlying its pathology remain poorly understood. Clarification of the molecular mechanisms involved in their development and/or treatment resistance is highly required. High mobility group box 1 protein (HMGB1) is a nuclear protein that can also act as an extracellular trigger of inflammation, proliferation and migration, through receptor for advanced glycation end products and toll like receptors in a number of cancers including gliomas. It is known that excessive release of HMGB1 in cancer leads to unlimited replicative potential, ability to develop blood vessels (angiogenesis), evasion of programmed cell death (apoptosis), self-sufficiency in growth signals, insensitivity to inhibitors of growth, inflammation, tissue invasion and metastasis. In this review we explore the mechanisms by which HMGB1 regulates apoptosis and autophagy in glioma. We also looked at how HMGB1 mediates glioma regression and promotes angiogenesis as well as possible signaling pathways with an attempt to provide potential therapeutic targets for the treatment of glioma.
Keywords: HMGB1; apoptosis; autophagy; glioma; macrophages.
Figures
References
-
- He J, Shan Z, Li L, et al. Expression of glioma stem cell marker CD133 and O6-methylguanine-DNA methyltransferase is associated with resistance to radiotherapy in gliomas. Oncol Rep 2011;26:1305. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

