Candida parapsilosis Protects Premature Intestinal Epithelial Cells from Invasion and Damage by Candida albicans
- PMID: 28382297
- PMCID: PMC5360698
- DOI: 10.3389/fped.2017.00054
Candida parapsilosis Protects Premature Intestinal Epithelial Cells from Invasion and Damage by Candida albicans
Abstract
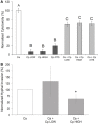
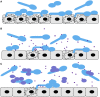
Candida is a leading cause of late-onset sepsis in premature infants and is thought to invade the host via immature or damaged epithelial barriers. We previously showed that the hyphal form of Candida albicans invades and causes damage to premature intestinal epithelial cells (pIECs), whereas the non-hyphal Candida parapsilosis, also a fungal pathogen of neonates, has less invasion and damage abilities. In this study, we investigated the potential for C. parapsilosis to modulate pathogenic interactions of C. albicans with the premature intestine. While a mixed infection with two fungal pathogens may be expected to result in additive or synergistic damage to pIECs, we instead found that C. parapsilosis was able to protect pIECs from invasion and damage by C. albicans. C. albicans-induced pIEC damage was reduced to a similar extent by multiple different C. parapsilosis strains, but strains differed in their ability to inhibit C. albicans invasion of pIECs, with the inhibitory activity correlating with their adhesiveness for C. albicans and epithelial cells. C. parapsilosis cell-free culture fractions were also able to significantly reduce C. albicans adhesion and damage to pIECs. Furthermore, coadministration of C. parapsilosis cell-free fractions with C. albicans was associated with decreased infection and mortality in zebrafish. These results indicate that C. parapsilosis is able to reduce invasion, damage, and virulence functions of C. albicans. Additionally, the results with cellular and cell-free fractions of yeast cultures suggest that inhibition of pathogenic interactions between C. albicans and host cells by C. parapsilosis occurs via secreted molecules as well as by physical contact with the C. parapsilosis cell surface. We propose that non-invasive commensals can be used to inhibit virulence features of pathogens and deserve further study as a non-pharmacological strategy to protect the fragile epithelial barriers of premature infants.
Keywords: Candida albicans; Candida parapsilosis; fungal pathogenesis; intestinal epithelium; premature infant; zebrafish model system.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

