Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade
- PMID: 28382931
- PMCID: PMC5384228
- DOI: 10.1038/ncomms14979
Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade
Abstract
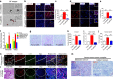
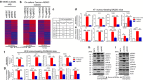
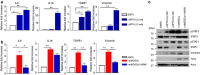
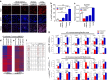
It is widely accepted that dynamic and reversible tumour cell plasticity is required for metastasis, however, in vivo steps and molecular mechanisms are poorly elucidated. We demonstrate here that monocytic (mMDSC) and granulocytic (gMDSC) subsets of myeloid-derived suppressor cells infiltrate in the primary tumour and distant organs with different time kinetics and regulate spatiotemporal tumour plasticity. Using co-culture experiments and mouse transcriptome analyses in syngeneic mouse models, we provide evidence that tumour-infiltrated mMDSCs facilitate tumour cell dissemination from the primary site by inducing EMT/CSC phenotype. In contrast, pulmonary gMDSC infiltrates support the metastatic growth by reverting EMT/CSC phenotype and promoting tumour cell proliferation. Furthermore, lung-derived gMDSCs isolated from tumour-bearing animals enhance metastatic growth of already disseminated tumour cells. MDSC-induced 'metastatic gene signature' derived from murine syngeneic model predicts poor patient survival in the majority of human solid tumours. Thus spatiotemporal MDSC infiltration may have clinical implications in tumour progression.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Husemann Y. et al.. Systemic spread is an early step in breast cancer. Cancer Cell 13, 58–68 (2008). - PubMed
-
- Nguyen D. X., Bos P. D. & Massague J. Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer 9, 274–284 (2009). - PubMed
-
- Klein C. A. Parallel progression of primary tumours and metastases. Nat. Rev. Cancer 9, 302–312 (2009). - PubMed
-
- Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastat. Rev. 8, 98–101 (1989). - PubMed
-
- Fidler I. J. Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res. 38, 2651–2660 (1978). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

