Diversity of Precursor Lesions For Pancreatic Cancer: The Genetics and Biology of Intraductal Papillary Mucinous Neoplasm
- PMID: 28383565
- PMCID: PMC5415899
- DOI: 10.1038/ctg.2017.3
Diversity of Precursor Lesions For Pancreatic Cancer: The Genetics and Biology of Intraductal Papillary Mucinous Neoplasm
Abstract
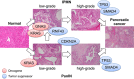
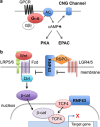
Pancreatic ductal adenocarcinoma (PDA), one of the most lethal cancers worldwide, is associated with two main types of morphologically distinct precursors-pancreatic intraepithelial neoplasia (PanIN) and intraductal papillary mucinous neoplasm (IPMN). Although the progression of PanIN into invasive cancer has been well characterized, there remains an urgent need to understand the biology of IPMNs, which are larger radiographically detectable cystic tumors. IPMNs comprise a number of subtypes with heterogeneous histopathologic and clinical features. Although frequently remaining benign, a significant proportion exhibits malignant progression. Unfortunately, there are presently no accurate prognosticators for assessing cancer risk in individuals with IPMN. Moreover, the fundamental mechanisms differentiating PanIN and IPMN remain largely obscure, as do those that distinguish IPMN subtypes. Recent studies, however, have identified distinct genetic profiles between PanIN and IPMN, providing a framework to better understand the diversity of the precursors for PDA. Here, we review the clinical, biological, and genetic properties of IPMN and discuss various models for progression of these tumors to invasive PDA.
Conflict of interest statement
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

