Neural Reorganization Due to Neonatal Amygdala Lesions in the Rhesus Monkey: Changes in Morphology and Network Structure
- PMID: 28383709
- PMCID: PMC6059167
- DOI: 10.1093/cercor/bhx080
Neural Reorganization Due to Neonatal Amygdala Lesions in the Rhesus Monkey: Changes in Morphology and Network Structure
Abstract
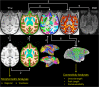
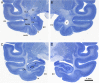
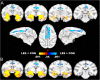
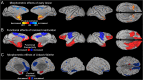
It is generally believed that neural damage that occurs early in development is associated with greater adaptive capacity relative to similar damage in an older individual. However, few studies have surveyed whole brain changes following early focal damage. In this report, we employed multimodal magnetic resonance imaging analyses of adult rhesus macaque monkeys who had previously undergone bilateral, neurotoxic lesions of the amygdala at about 2 weeks of age. A deformation-based morphometric approach demonstrated reduction of the volumes of the anterior temporal lobe, anterior commissure, basal ganglia, and pulvinar in animals with early amygdala lesions compared to controls. In contrast, animals with early amygdala lesions had an enlarged cingulate cortex, medial superior frontal gyrus, and medial parietal cortex. Diffusion-weighted imaging tractography and network analysis were also used to compare connectivity patterns and higher-level measures of communication across the brain. Using the communicability metric, which integrates direct and indirect paths between regions, lesioned animals showed extensive degradation of network integrity in the temporal and orbitofrontal cortices. This work demonstrates both degenerative as well as progressive large-scale neural changes following long-term recovery from neonatal focal brain damage.
Keywords: brain damage; connectome; morphometry; plasticity; tractography.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures



Similar articles
-
Do neonatal bilateral ibotenic acid lesions of the hippocampal formation or of the amygdala impair HPA axis responsiveness and regulation in infant rhesus macaques (Macaca mulatta)?Brain Res. 2006 Feb 3;1071(1):97-104. doi: 10.1016/j.brainres.2005.11.027. Epub 2006 Jan 17. Brain Res. 2006. PMID: 16412391
-
Effects of neonatal amygdala or hippocampus lesions on resting brain metabolism in the macaque monkey: a microPET imaging study.Neuroimage. 2008 Jan 15;39(2):832-46. doi: 10.1016/j.neuroimage.2007.09.029. Epub 2007 Sep 26. Neuroimage. 2008. PMID: 17964814 Free PMC article.
-
The effective connectivity of the seizure onset zone and ictal perfusion changes in amygdala kindled rhesus monkeys.Neuroimage Clin. 2016 Jun 1;12:252-61. doi: 10.1016/j.nicl.2016.05.020. eCollection 2016. Neuroimage Clin. 2016. PMID: 27489773 Free PMC article.
-
The expression of social dominance following neonatal lesions of the amygdala or hippocampus in rhesus monkeys (Macaca mulatta).Behav Neurosci. 2006 Aug;120(4):749-60. doi: 10.1037/0735-7044.120.4.749. Behav Neurosci. 2006. PMID: 16893283
-
An MRI study of the corpus callosum in monkeys: Developmental trajectories and effects of neonatal hippocampal and amygdala lesions.Dev Psychobiol. 2017 May;59(4):495-506. doi: 10.1002/dev.21514. Epub 2017 Apr 3. Dev Psychobiol. 2017. PMID: 28369850 Free PMC article.
Cited by
-
Anterior Cingulate Cortex Ablation Disrupts Affective Vigor and Vigilance.J Neurosci. 2021 Sep 22;41(38):8075-8087. doi: 10.1523/JNEUROSCI.0673-21.2021. Epub 2021 Aug 11. J Neurosci. 2021. PMID: 34380767 Free PMC article.
-
Neuroanatomical abnormalities in a nonhuman primate model of congenital Zika virus infection.Elife. 2022 Mar 9;11:e64734. doi: 10.7554/eLife.64734. Elife. 2022. PMID: 35261339 Free PMC article.
-
Reorganization in the macaque interoceptive-allostatic network following anterior cingulate cortex damage.Cereb Cortex. 2023 Apr 4;33(8):4334-4349. doi: 10.1093/cercor/bhac346. Cereb Cortex. 2023. PMID: 36066407 Free PMC article.
-
Amygdala or hippocampus damage only minimally impacts affective responding to threat.Behav Neurosci. 2022 Feb;136(1):30-45. doi: 10.1037/bne0000491. Epub 2021 Oct 7. Behav Neurosci. 2022. PMID: 34618493 Free PMC article.
-
Four Social Brain Regions, Their Dysfunctions, and Sequelae, Extensively Explain Autism Spectrum Disorder Symptomatology.Brain Sci. 2019 Jun 4;9(6):130. doi: 10.3390/brainsci9060130. Brain Sci. 2019. PMID: 31167459 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

