Gene Therapy 2017: Progress and Future Directions
- PMID: 28383804
- PMCID: PMC5504480
- DOI: 10.1111/cts.12466
Gene Therapy 2017: Progress and Future Directions
Figures
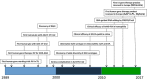

References
-
- Rosenberg, S.A. , Aebersold, P. , Cornetta, K. , Kasid, A. , Morgan, R.A. , Moen, R. et al Gene transfer into humans–immunotherapy of patients with advanced melanoma, using tumor‐infiltrating lymphocytes modified by retroviral gene transduction. N. Engl. J. Med. 323, 570–578 (1990). - PubMed
-
- San, H. , Yang, Z.Y. , Pompili, V.J. , Jaffe, M.L. , Plautz, G.E. , Xu, L. et al Safety and short‐term toxicity of a novel cationic lipid formulation for human gene therapy. Hum. Gene Ther. 4, 781–788 (1993). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

