Clinical Significance of Androgen Receptor Splice Variant-7 mRNA Detection in Circulating Tumor Cells of Men With Metastatic Castration-Resistant Prostate Cancer Treated With First- and Second-Line Abiraterone and Enzalutamide
- PMID: 28384066
- PMCID: PMC5493048
- DOI: 10.1200/JCO.2016.70.1961
Clinical Significance of Androgen Receptor Splice Variant-7 mRNA Detection in Circulating Tumor Cells of Men With Metastatic Castration-Resistant Prostate Cancer Treated With First- and Second-Line Abiraterone and Enzalutamide
Abstract
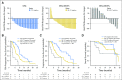
Purpose We reported previously that the detection of androgen receptor splice variant-7 (AR-V7) mRNA in circulating tumor cells (CTCs) correlated with poor outcomes from the use of abiraterone and enzalutamide in patients with castration-resistant prostate cancer (CRPC). Here, we expanded our cohort size to better characterize the prognostic significance of AR-V7 in this setting. Methods We prospectively enrolled 202 patients with CRPC starting abiraterone or enzalutamide and investigated the prognostic value of CTC detection (+ v -) and AR-V7 detection (+ v -) using a CTC-based AR-V7 mRNA assay. We examined ≥ 50% prostate-specific antigen (PSA) responses, PSA progression-free survival, clinical and radiologic progression-free survival, and overall survival. We constructed multivariable models adjusting for PSA, Gleason sum, number of prior hormone therapies, prior abiraterone or enzalutamide use, prior taxane use, presence of visceral metastases, and Eastern Cooperative Oncology Group score. We also separately examined the first-line and second-line novel hormonal therapy (NHT) settings. Results Median follow-up times were 15.0, 21.7, and 14.6 months for CTC-, CTC+/AR-V7- and CTC+/AR-V7+ patients, respectively. CTC+/AR-V7+ patients were more likely to have Gleason scores ≥ 8 ( P = .05), metastatic disease at diagnosis ( P = .01), higher PSA ( P < .01), prior abiraterone or enzalutamide use ( P = .03), prior taxane use ( P = .02), and Eastern Cooperative Oncology Group ≥ 1 ( P = .01). Outcomes for the overall cohort (and separately for the first-line and second-line NHT cohorts) were best for CTC- patients, intermediate for CTC+/AR-V7- patients, and worse for CTC+/AR-V7+ patients. These correlations remained significant in multivariable models. Conclusion This expanded analysis further characterizes the importance of CTC-based AR-V7 mRNA detection in predicting outcomes in patients with CRPC receiving first- and second-line NHT and, to the best of our knowledge, is the first to suggest that this assay be interpreted using three separate prognostic categories: CTC-, CTC+/AR-V7-, and CTC+/AR-V7+.
Figures


Comment in
-
Association Between Androgen Receptor Splice Variants and Prostate Cancer Resistance to Abiraterone and Enzalutamide.J Clin Oncol. 2017 Jul 1;35(19):2103-2105. doi: 10.1200/JCO.2017.72.8808. Epub 2017 Apr 17. J Clin Oncol. 2017. PMID: 28414609 No abstract available.
-
Re: Emmanuel S. Antonarakis, Changxue Lu, Brandon Luber, et al. Clinical Significance of Androgen Receptor Splice Variant-7 mRNA Detection in Circulating Tumor Cells of Men with Metastatic Castration-resistant Prostate Cancer Treated with First- and Second-line Abiraterone and Enzalutamide. J Clin Oncol 2017;35:2149-56: AR-V7 Testing: What's in it for the Patient?Eur Urol. 2017 Dec;72(6):e168-e169. doi: 10.1016/j.eururo.2017.06.031. Epub 2017 Jul 5. Eur Urol. 2017. PMID: 28688614 No abstract available.
-
Reply to Julie Steinestel, Christof Bernemann, Andres J. Schrader, and Jochen K. Lennerz's Letter to the Editor re: Emmanuel S. Antonarakis, Changxue Lu, Brandon Luber, et al. Clinical Significance of Androgen Receptor Splice Variant-7 mRNA Detection in Circulating Tumor Cells of Men with Metastatic Castration-resistant Prostate Cancer Treated with First- and Second-line Abiraterone and Enzalutamide. J Clin Oncol 2017;35:2149-56. AR-V7 Testing: What's in it for the Patient?: Estimating the Clinical Utility of Blood-based AR-V7 Testing in Prostate Cancer.Eur Urol. 2017 Dec;72(6):e170-e171. doi: 10.1016/j.eururo.2017.06.032. Epub 2017 Jul 8. Eur Urol. 2017. PMID: 28693939 No abstract available.
References
-
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. - PubMed
-
- Steinestel J, Luedeke M, Arndt A, et al: Detecting predictive androgen receptor modifications in circulating prostate cancer cells. Oncotarget 10.18632/oncotarget.3925 [epub ahead of print on April 23, 2015] - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

