Deciphering neuronal population codes for acute thermal pain
- PMID: 28384122
- PMCID: PMC5679238
- DOI: 10.1088/1741-2552/aa644d
Deciphering neuronal population codes for acute thermal pain
Abstract
Objective: Pain is defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage. Current pain research mostly focuses on molecular and synaptic changes at the spinal and peripheral levels. However, a complete understanding of pain mechanisms requires the physiological study of the neocortex. Our goal is to apply a neural decoding approach to read out the onset of acute thermal pain signals, which can be used for brain-machine interface.
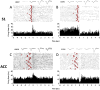
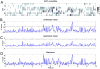
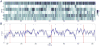
Approach: We used micro wire arrays to record ensemble neuronal activities from the primary somatosensory cortex (S1) and anterior cingulate cortex (ACC) in freely behaving rats. We further investigated neural codes for acute thermal pain at both single-cell and population levels. To detect the onset of acute thermal pain signals, we developed a novel latent state-space framework to decipher the sorted or unsorted S1 and ACC ensemble spike activities, which reveal information about the onset of pain signals.
Main results: The state space analysis allows us to uncover a latent state process that drives the observed ensemble spike activity, and to further detect the 'neuronal threshold' for acute thermal pain on a single-trial basis. Our method achieved good detection performance in sensitivity and specificity. In addition, our results suggested that an optimal strategy for detecting the onset of acute thermal pain signals may be based on combined evidence from S1 and ACC population codes.
Significance: Our study is the first to detect the onset of acute pain signals based on neuronal ensemble spike activity. It is important from a mechanistic viewpoint as it relates to the significance of S1 and ACC activities in the regulation of the acute pain onset.
Figures


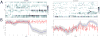





References
-
- Armstrong-James M, George MJ. Bilateral receptive fields of cells in rat Sm1 cortex. Exp Brain Res. 1988;70:155–65. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
