T/T homozygosity of the tenascin-C gene polymorphism rs2104772 negatively influences exercise-induced angiogenesis
- PMID: 28384286
- PMCID: PMC5383042
- DOI: 10.1371/journal.pone.0174864
T/T homozygosity of the tenascin-C gene polymorphism rs2104772 negatively influences exercise-induced angiogenesis
Abstract
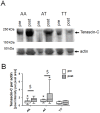
Background: Mechanical stress, including blood pressure related factors, up-regulate expression of the pro-angiogenic extracellular matrix protein tenascin-C in skeletal muscle. We hypothesized that increased capillarization of skeletal muscle with the repeated augmentation in perfusion during endurance training is associated with blood vessel-related expression of tenascin-C and would be affected by the single-nucleotide polymorphism (SNP) rs2104772, which characterizes the non-synonymous exchange of thymidine (T)-to-adenosine (A) in the amino acid codon 1677 of tenascin-C.
Methods: Sixty-one healthy, untrained, male white participants of Swiss descent performed thirty 30-min bouts of endurance exercise on consecutive weekdays using a cycling ergometer. Genotype and training interactions were called significant at Bonferroni-corrected p-value of 5% (repeated measures ANOVA).
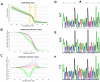
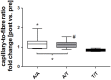
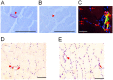
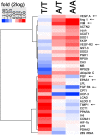
Results: Endurance training increased capillary-to-fiber-ratio (+11%), capillary density (+7%), and mitochondrial volume density (+30%) in m. vastus lateralis. Tenascin-C protein expression in this muscle was confined to arterioles and venules (80% of cases) and increased after training in A-allele carriers. Prior to training, volume densities of subsarcolemmal and myofibrillar mitochondria in m. vastus lateralis muscle were 49% and 18%, respectively, higher in A/A homozygotes relative to T-nucleotide carriers (A/T and T/T). Training specifically increased capillary-to-fiber ratio in A-nucleotide carriers but not in T/T homozygotes. Genotype specific regulation of angiogenesis was reflected by the expression response of 8 angiogenesis-associated transcripts after exercise, and confirmed by training-induced alterations of the shear stress related factors, vimentin and VEGF A.
Conclusion: Our findings provide evidence for a negative influence of T/T homozygosity in rs2104772 on capillary remodeling with endurance exercise.
Conflict of interest statement
Figures


Similar articles
-
The Metabolic Response of Skeletal Muscle to Endurance Exercise Is Modified by the ACE-I/D Gene Polymorphism and Training State.Front Physiol. 2017 Dec 14;8:993. doi: 10.3389/fphys.2017.00993. eCollection 2017. Front Physiol. 2017. PMID: 29311951 Free PMC article.
-
Cellular Aspects of Muscle Specialization Demonstrate Genotype - Phenotype Interaction Effects in Athletes.Front Physiol. 2019 May 8;10:526. doi: 10.3389/fphys.2019.00526. eCollection 2019. Front Physiol. 2019. PMID: 31139091 Free PMC article.
-
Angiogenesis-related ultrastructural changes to capillaries in human skeletal muscle in response to endurance exercise.J Appl Physiol (1985). 2015 Nov 15;119(10):1118-26. doi: 10.1152/japplphysiol.00594.2015. Epub 2015 Sep 17. J Appl Physiol (1985). 2015. PMID: 26384412
-
Endurance Exercise and the Regulation of Skeletal Muscle Metabolism.Prog Mol Biol Transl Sci. 2015;135:129-51. doi: 10.1016/bs.pmbts.2015.07.016. Epub 2015 Sep 5. Prog Mol Biol Transl Sci. 2015. PMID: 26477913 Review.
-
Angiogenesis during exercise and training.Angiogenesis. 2005;8(3):263-71. doi: 10.1007/s10456-005-9013-x. Epub 2005 Nov 19. Angiogenesis. 2005. PMID: 16328159 Review.
Cited by
-
Exercise Training-Induced Extracellular Matrix Protein Adaptation in Locomotor Muscles: A Systematic Review.Cells. 2021 Apr 26;10(5):1022. doi: 10.3390/cells10051022. Cells. 2021. PMID: 33926070 Free PMC article.
-
The Metabolic Response of Skeletal Muscle to Endurance Exercise Is Modified by the ACE-I/D Gene Polymorphism and Training State.Front Physiol. 2017 Dec 14;8:993. doi: 10.3389/fphys.2017.00993. eCollection 2017. Front Physiol. 2017. PMID: 29311951 Free PMC article.
-
A glitch in the matrix: the pivotal role for extracellular matrix remodeling during muscle hypertrophy.Am J Physiol Cell Physiol. 2022 Sep 1;323(3):C763-C771. doi: 10.1152/ajpcell.00200.2022. Epub 2022 Jul 25. Am J Physiol Cell Physiol. 2022. PMID: 35876284 Free PMC article. Review.
-
Does a Better Perfusion of Deconditioned Muscle Tissue Release Chronic Low Back Pain?Front Med (Lausanne). 2018 Mar 20;5:77. doi: 10.3389/fmed.2018.00077. eCollection 2018. Front Med (Lausanne). 2018. PMID: 29616222 Free PMC article.
-
Genotypic Influences on Actuators of Aerobic Performance in Tactical Athletes.Genes (Basel). 2024 Nov 28;15(12):1535. doi: 10.3390/genes15121535. Genes (Basel). 2024. PMID: 39766802 Free PMC article.
References
-
- Mathes S, van Ginkel S, Vaughan D, Valdivieso P, Flück M. Gene-Pharmacologial Effects on Exercise-Induced Muscle Gene Expression in Healthy Men. Anat Physiol (2015); S5:005.
-
- Imanaka-Yoshida K, Yoshida T, Miyagawa-Tomita S. Tenascin-C in development and disease of blood vessels. Anat Rec (Hoboken). 2014;297(9):1747–57. Epub 2014/08/16. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

