Polyglutamine-Expanded Huntingtin Exacerbates Age-Related Disruption of Nuclear Integrity and Nucleocytoplasmic Transport
- PMID: 28384474
- PMCID: PMC5479704
- DOI: 10.1016/j.neuron.2017.03.027
Polyglutamine-Expanded Huntingtin Exacerbates Age-Related Disruption of Nuclear Integrity and Nucleocytoplasmic Transport
Abstract
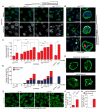
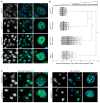
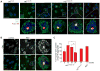
Onset of neurodegenerative disorders, including Huntington's disease, is strongly influenced by aging. Hallmarks of aged cells include compromised nuclear envelope integrity, impaired nucleocytoplasmic transport, and accumulation of DNA double-strand breaks. We show that mutant huntingtin markedly accelerates all of these cellular phenotypes in a dose- and age-dependent manner in cortex and striatum of mice. Huntingtin-linked polyglutamine initially accumulates in nuclei, leading to disruption of nuclear envelope architecture, partial sequestration of factors essential for nucleocytoplasmic transport (Gle1 and RanGAP1), and intranuclear accumulation of mRNA. In aged mice, accumulation of RanGAP1 together with polyglutamine is shifted to perinuclear and cytoplasmic areas. Consistent with findings in mice, marked alterations in nuclear envelope morphology, abnormal localization of RanGAP1, and nuclear accumulation of mRNA were found in cortex of Huntington's disease patients. Overall, our findings identify polyglutamine-dependent inhibition of nucleocytoplasmic transport and alteration of nuclear integrity as a central component of Huntington's disease.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

References
-
- Bennett EJ, Shaler TA, Woodman B, Ryu KY, Zaitseva TS, Becker CH, Bates GP, Schulman H, Kopito RR. Global changes to the ubiquitin system in Huntington’s disease. Nature. 2007;448:704–708. - PubMed
-
- Bukata L, Parker SL, D’angelo MA. Nuclear pore complexes in the maintenance of genome integrity. Curr Opin Cell Biol. 2013;25:378–386. - PubMed
-
- Carty N, Berson N, Tillack K, Thiede C, Scholz D, Kottig K, Sedaghat Y, Gabrysiak C, Yohrling G, Von Der Kammer H, et al. Characterization of HTT inclusion size, location, and timing in the zQ175 mouse model of Huntington’s disease: an in vivo high-content imaging study. PLoS One. 2015;10:e0123527. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

