Mutant Huntingtin Disrupts the Nuclear Pore Complex
- PMID: 28384479
- PMCID: PMC5595097
- DOI: 10.1016/j.neuron.2017.03.023
Mutant Huntingtin Disrupts the Nuclear Pore Complex
Abstract
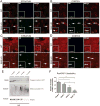
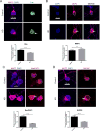
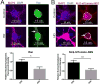
Huntington's disease (HD) is caused by an expanded CAG repeat in the Huntingtin (HTT) gene. The mechanism(s) by which mutant HTT (mHTT) causes disease is unclear. Nucleocytoplasmic transport, the trafficking of macromolecules between the nucleus and cytoplasm, is tightly regulated by nuclear pore complexes (NPCs) made up of nucleoporins (NUPs). Previous studies offered clues that mHTT may disrupt nucleocytoplasmic transport and a mutation of an NUP can cause HD-like pathology. Therefore, we evaluated the NPC and nucleocytoplasmic transport in multiple models of HD, including mouse and fly models, neurons transfected with mHTT, HD iPSC-derived neurons, and human HD brain regions. These studies revealed severe mislocalization and aggregation of NUPs and defective nucleocytoplasmic transport. HD repeat-associated non-ATG (RAN) translation proteins also disrupted nucleocytoplasmic transport. Additionally, overexpression of NUPs and treatment with drugs that prevent aberrant NUP biology also mitigated this transport defect and neurotoxicity, providing future novel therapy targets.
Keywords: C9ORF72; Huntington’s disease; KPT-350; O-GlcNAc; RAN translation; Thiamet-G; induced pluripotent stem cell; neurodegeneration; nuclear pore complex; nucleocytoplasmic transport.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
None of the authors have competing financial interests.
Figures





References
-
- Basel-Vanagaite L, Muncher L, Straussberg R, Pasmanik-Chor M, Yahav M, Rainshtein L, Walsh CA, Magal N, Taub E, Drasinover V, et al. Mutated nup62 causes autosomal recessive infantile bilateral striatal necrosis. Ann Neurol. 2006;60:214–222. - PubMed
MeSH terms
Substances
Grants and funding
- R01 NS082338/NS/NINDS NIH HHS/United States
- T32 GM007309/GM/NIGMS NIH HHS/United States
- R01 NS085207/NS/NINDS NIH HHS/United States
- F31 NS103360/NS/NINDS NIH HHS/United States
- R21 NS099670/NS/NINDS NIH HHS/United States
- U54 NS091046/NS/NINDS NIH HHS/United States
- R01 NS090390/NS/NINDS NIH HHS/United States
- R37 NS040389/NS/NINDS NIH HHS/United States
- R01 NS094239/NS/NINDS NIH HHS/United States
- R01 NS086452/NS/NINDS NIH HHS/United States
- P50 AG005146/AG/NIA NIH HHS/United States
- R21 NS094886/NS/NINDS NIH HHS/United States
- P01 NS058901/NS/NINDS NIH HHS/United States
- T32 GM008752/GM/NIGMS NIH HHS/United States
- R01 NS098819/NS/NINDS NIH HHS/United States
- P01 HL107153/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

