In vitro antileishmanial activity of fisetin flavonoid via inhibition of glutathione biosynthesis and arginase activity in Leishmania infantum
- PMID: 28385129
- PMCID: PMC5498762
- DOI: 10.1080/20477724.2017.1312777
In vitro antileishmanial activity of fisetin flavonoid via inhibition of glutathione biosynthesis and arginase activity in Leishmania infantum
Abstract
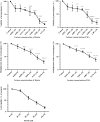
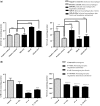
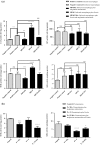
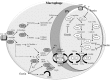
With the increasing emergence of drug resistant Leishmania sp. in recent years, combination therapy has been considered as a useful way to treat and control of Leishmaniasis. The present study was designed to evaluate the antileishmanial effects of the fisetin alone and combination of fisetin plus Meglumine antimoniate (Fi-MA) against Leishmania infantum. The IC50 values for fisetin were obtained 0.283 and 0.102 μM against promastigotes and amastigote forms, respectively. Meglumine antimoniate (MA, Glucantime) as control drug also revealed IC50 values of 0.247 and 0.105 μM for promastigotes and amastigotes of L. infantum, respectively. In order to determine the mode of action of fisetin and Meglumine antimoniate (MA, Glucantime), the activities of arginase (ARG), catalase (CAT), glutathione peroxidase (GPx) and superoxide dismutase (SOD) were measured. Moreover, intracellular glutathione (GSH) and nitric oxide (NO) levels in L. infantum-infected macrophages and L. infantum promastigotes which were treated with IC50 concentrations of fisetin, MA and Fi-MA were investigated. Our results showed that MA decreased CAT and SOD activity and increased NO levels in L. infantum-infected macrophages. In promastigotes, MA inhibited parasite SOD activity and reduced parasite NO production. The decreased levels of most of the antioxidant enzymes, accompanying by the raised level of NO in treated macrophages with MA, were observed to regain their normal profiles due to Fi-MA treatment. Furthermore, fisetin could prevent the growth of promastigotes by inhibition of ARG activity and reduction of GSH levels and NO production. In conclusion, these findings showed that fisetin improves MA side effects.
Keywords: Leishmania infantum; antioxidant enzymes; arginase; fisetin; glutathione; nitric oxide.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
