Phosphatidylcholine transfer protein/StarD2 promotes microvesicular steatosis and liver injury in murine experimental steatohepatitis
- PMID: 28385694
- PMCID: PMC5538832
- DOI: 10.1152/ajpgi.00379.2016
Phosphatidylcholine transfer protein/StarD2 promotes microvesicular steatosis and liver injury in murine experimental steatohepatitis
Abstract
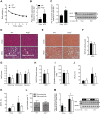
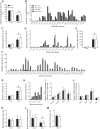
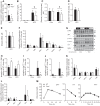
Mice fed a methionine- and choline-deficient (MCD) diet develop steatohepatitis that recapitulates key features of nonalcoholic steatohepatitis (NASH) in humans. Phosphatidylcholine is the most abundant phospholipid in the surfactant monolayer that coats and stabilizes lipid droplets within cells, and choline is required for its major biosynthetic pathway. Phosphatidylcholine-transfer protein (PC-TP), which exchanges phosphatidylcholines among membranes, is enriched in hepatocytes. PC-TP also regulates fatty acid metabolism through interactions with thioesterase superfamily member 2. We investigated the contribution of PC-TP to steatohepatitis induced by the MCD diet. Pctp-/- and wild-type control mice were fed the MCD diet for 5 wk and were then euthanized for histopathologic and biochemical analyses, as well as determinations of mRNA and protein expression. Whereas all mice developed steatohepatitis, plasma alanine aminotransferase and aspartate aminotransferase activities were only elevated in wild-type mice, indicating that Pctp-/- mice were protected from MCD diet-induced hepatocellular injury. Reduced hepatotoxicity due to the MCD diet in the absence of PC-TP expression was further evidenced by decreased activation of c-Jun and reduced plasma concentrations of fibroblast growth factor 21. Despite similar total hepatic concentrations of phosphatidylcholines and other lipids, the relative abundance of microvesicular lipid droplets within hepatocytes was reduced in Pctp-/- mice. Considering that the formation of larger lipid droplets may serve to protect against lipotoxicity in NASH, our findings suggest a pathogenic role for PC-TP that could be targeted in the management of this condition.NEW & NOTEWORTHY Phosphatidylcholine-transfer protein (PC-TP) is a highly specific phosphatidylcholine-binding protein that we previously showed to regulate hepatocellular nutrient metabolism through its interacting partner thioesterase superfamily member 2 (Them2). This study identifies a pathogenic role for PC-TP, independent of Them2, in the methionine- and choline-deficient diet model of experimental steatohepatitis. Our current observations suggest that PC-TP promotes liver injury by mediating the intermembrane transfer of phosphatidylcholines, thus stabilizing more pathogenic microvesicular lipid droplets.
Keywords: lipid droplet; methionine- and choline-deficient diet; nonalcoholic steatohepatitis; phospholipid; triglyceride.
Copyright © 2017 the American Physiological Society.
Figures


References
-
- Carpino G, Morini S, Ginanni Corradini S, Franchitto A, Merli M, Siciliano M, Gentili F, Onetti Muda A, Berloco P, Rossi M, Attili AF, Gaudio E. Alpha-SMA expression in hepatic stellate cells and quantitative analysis of hepatic fibrosis in cirrhosis and in recurrent chronic hepatitis after liver transplantation. Dig Liver Dis 37: 349–356, 2005. doi:10.1016/j.dld.2004.11.009. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

