Microbial Immuno-Communication in Neurodegenerative Diseases
- PMID: 28386215
- PMCID: PMC5362619
- DOI: 10.3389/fnins.2017.00151
Microbial Immuno-Communication in Neurodegenerative Diseases
Abstract
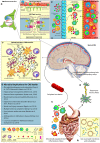
Neuro-inflammation is a critical process by which the brain coordinates chemokine-regulated cellular recruitment, cytokine release, and cell-mediated removal of pathogenic material to protect against infection or brain injury. Dysregulation of this immune response is involved in multiple neurodegenerative disorders, however the precise contribution of neuro-inflammation to the exacerbation and progression of these diseases remains unclear. Evidence now suggests that commensal micro-organisms populating the host and their metabolites, collectively termed the microbiome, regulate innate immunity by influencing peripheral immune cell populations, and modulating microglial phenotype. Recent preclinical studies now demonstrate that perturbations in the host microbiome can induce alterations in pathological phenotypes associated with numerous neurodegenerative diseases. How perturbations in the host microbiome and subsequently altered peripheral immune status are communicated to the brain to influence neuro-inflammatory processes in these neurodegenerative disease settings is far from understood. This review provides insight into the regulation of neuro-inflammatory processes by the host microbiome in the context of neurodegenerative disease and highlights the potential importance of the blood-brain barrier and blood-cerebrospinal fluid-brain barrier, functioning as "immune barriers," to communicate host immune status to the brain. Understanding the mechanisms by which the commensal microbiome communicates with the brain to influence neuro-inflammatory processes will be critical in the development of microbially-targeted therapeutics in the potential treatment of neurodegenerative disorders.
Keywords: Alzheimer's disease; Parkinson's disease; blood brain barrier; blood cerebrospinal fluid-brain barrier; immunity; microbiome; neuro-inflammation; stroke.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

