ClC Channels and Transporters: Structure, Physiological Functions, and Implications in Human Chloride Channelopathies
- PMID: 28386229
- PMCID: PMC5362633
- DOI: 10.3389/fphar.2017.00151
ClC Channels and Transporters: Structure, Physiological Functions, and Implications in Human Chloride Channelopathies
Abstract
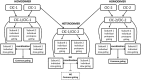
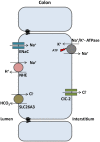
The discovery of ClC proteins at the beginning of the 1990s was important for the development of the Cl- transport research field. ClCs form a large family of proteins that mediate voltage-dependent transport of Cl- ions across cell membranes. They are expressed in both plasma and intracellular membranes of cells from almost all living organisms. ClC proteins form transmembrane dimers, in which each monomer displays independent ion conductance. Eukaryotic members also possess a large cytoplasmic domain containing two CBS domains, which are involved in transport modulation. ClC proteins function as either Cl- channels or Cl-/H+ exchangers, although all ClC proteins share the same basic architecture. ClC channels have two gating mechanisms: a relatively well-studied fast gating mechanism, and a slow gating mechanism, which is poorly defined. ClCs are involved in a wide range of physiological processes, including regulation of resting membrane potential in skeletal muscle, facilitation of transepithelial Cl- reabsorption in kidneys, and control of pH and Cl- concentration in intracellular compartments through coupled Cl-/H+ exchange mechanisms. Several inherited diseases result from C1C gene mutations, including myotonia congenita, Bartter's syndrome (types 3 and 4), Dent's disease, osteopetrosis, retinal degeneration, and lysosomal storage diseases. This review summarizes general features, known or suspected, of ClC structure, gating and physiological functions. We also discuss biophysical properties of mammalian ClCs that are directly involved in the pathophysiology of several human inherited disorders, or that induce interesting phenotypes in animal models.
Keywords: ClC channels; Dent’s disease; channelopathy; deafness; leukodystrophy; myotonia congenita; osteopetrosis; salt loss.
Figures





References
-
- Adachi S., Uchida S., Ito H., Hata M., Hiroe M., Marumo F., et al. (1994). Two isoforms of a chloride channel predominantly expressed in thick ascending limb of Henle’s loop and collecting ducts of rat kidney. J. Biol. Chem. 269 17677–17683. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

