Identification of Ellagic Acid Rhamnoside as a Bioactive Component of a Complex Botanical Extract with Anti-biofilm Activity
- PMID: 28386254
- PMCID: PMC5362615
- DOI: 10.3389/fmicb.2017.00496
Identification of Ellagic Acid Rhamnoside as a Bioactive Component of a Complex Botanical Extract with Anti-biofilm Activity
Abstract
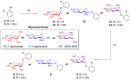
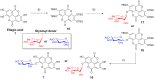
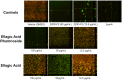
Staphylococcus aureus is a leading cause of hospital-acquired infections. It is listed among the top "serious threats" to human health in the USA, due in large part to rising rates of resistance. Many S. aureus infections are recalcitrant to antibiotic therapy due to their ability to form a biofilm, which acts not only as a physical barrier to antibiotics and the immune system, but results in differences in metabolism that further restricts antibiotic efficacy. Development of a modular strategy to synthesize a library of phenolic glycosides allowed for bioactivity testing and identification of anti-biofilm compounds within an extract of the elmleaf blackberry (Rubus ulmifolius). Two ellagic acid (EA) derivatives, EA xyloside and EA rhamnoside, have been identified as components of the Rubus extract. In addition, EA rhamnoside has been identified as an inhibitor of biofilm formation, with activity comparable to the complex extract 220D-F2 (composed of a mixture of EA glycosides), and confirmed by confocal laser scanning microscopy analyses.
Keywords: Rubus ulmifolius; Staphylococcus aureus; biofilm; ellagic acid; natural products.
Figures

References
-
- Baldoni D., Steinhuber A., Zimmerli W., Trampuz A. (2010). In vitro activity of gallium maltolate against staphylococci in logarithmic, stationary, and biofilm growth phases: comparison of conventional and calorimetric susceptibility testing methods. Antimicrob. Agents Chemother. 54 157–163. 10.1128/AAC.00700-09 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

