Celecoxib enhances the radiosensitivity of HCT116 cells in a COX-2 independent manner by up-regulating BCCIP
- PMID: 28386336
- PMCID: PMC5376001
Celecoxib enhances the radiosensitivity of HCT116 cells in a COX-2 independent manner by up-regulating BCCIP
Abstract
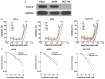
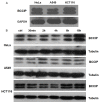
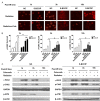
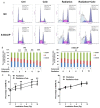
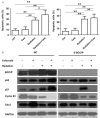
It has been reported that celecoxib, a cyclooxygenase-2 (COX-2)-selective nonsteroidal anti-inflammatory drug (NSAID), regulates the radiosensitivity of several cancer cells. BCCIP (BRCA2 and CDKN1A interacting protein) plays a critical role in maintaining the critical functions of p53 in tumor suppression and response to therapy. However, whether the effect of celecoxib on the radiosensitivity of colorectal cancer (CRC) cells is dependent on BCCIP is largely unclear. In this study, we found that celecoxib enhanced the radiosensitivity of HeLa (a human cervical carcinoma cell line), A549 (a human lung carcinoma cell line), and HCT116 cells (a human CRC cells line). Among these cells, COX-2 expression was undetected in HCT116 cells. Treatment with celecoxib significantly increased BCCIP expression in COX-2 negative HCT116 cells. Knockdown of BCCIP obviously abrogated the enhanced radiosensitivity of HCT116 cells induced by celecoxib. A combination of celecoxib and irradiation treatment induced much more γ-H2AX foci formation, higher levels of radiation injury-related proteins phosphorylation, G2/M arrest, apoptosis, and p53 and p21 expression, and lower levels of Cyclin B1 in HCT116 cells than those in cells treated with irradiation alone. However, these changes were undetected in BCCIP-silenced HCT116 cells. Therefore, these data suggest that BCCIP gene may be a radiosensitivity-related gene in CRC. Celecoxib affects the functions of p53 and inhibits the recovery from the irradiation-induced injury by up-regulating the expression of BCCIP, and subsequently regulates the expressions of genes such as p21 and Cyclin B1 to enhance the radiosensitivity of HCT116 cells in a COX-2 independent manner.
Keywords: BCCIP; COX-2; Celecoxib; colorectal cancer cells; radiosensitivity.
Figures

References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. - PubMed
-
- Improved survival with preoperative radiotherapy in respectable rectal cancer. Swedish rectal cancer trial. N Engl J Med. 1997;336:980–987. - PubMed
-
- Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC EORTC Radiotherapy Group Trial 22921. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. - PubMed
-
- Gerard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E, Maurel J, Seitz JF, Buecher B, Mackiewicz R, Ducreux M, Bedenne L. Preoperativeradiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J. Clin. Oncol. 2006;24:4620–4625. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
