Pellet-free isolation of human and bovine milk extracellular vesicles by size-exclusion chromatography
- PMID: 28386391
- PMCID: PMC5373680
- DOI: 10.1080/20013078.2017.1294340
Pellet-free isolation of human and bovine milk extracellular vesicles by size-exclusion chromatography
Abstract
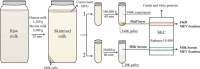
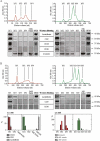
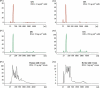
Studies have suggested that nanoscale extracellular vesicles (EV) in human and bovine milk carry immune modulatory properties which could provide beneficial health effects to infants. In order to assess the possible health effects of milk EV, it is essential to use isolates of high purity from other more abundant milk structures with well-documented bioactive properties. Furthermore, gentle isolation procedures are important for reducing the risk of generating vesicle artefacts, particularly when EV subpopulations are investigated. In this study, we present two isolation approaches accomplished in three steps based on size-exclusion chromatography (SEC) resulting in effective and reproducible EV isolation from raw milk. The approaches do not require any EV pelleting and can be applied to both human and bovine milk. We show that SEC effectively separates phospholipid membrane vesicles from the primary casein and whey protein components in two differently obtained casein reduced milk fractions, with one of the fractions obtained without the use of ultracentrifugation. Milk EV isolates were enriched in lactadherin, CD9, CD63 and CD81 compared to minimal levels of the EV-marker proteins in other relevant milk fractions such as milk fat globules. Nanoparticle tracking analysis and electron microscopy reveals the presence of heterogeneous sized vesicle structures in milk EV isolates. Lipid analysis by thin layer chromatography shows that EV isolates are devoid of triacylglycerides and presents a phospholipid profile differing from milk fat globules surrounded by epithelial cell plasma membrane. Moreover, the milk EV fractions are enriched in RNA with distinct and diverging profiles from milk fat globules. Collectively, our data supports that successful milk EV isolation can be accomplished in few steps without the use of ultracentrifugation, as the presented isolation approaches based on SEC effectively isolates EV in both human and bovine milk.
Keywords: EM; Exosomes; MFGM; RNA; phospholipids; size-exclusion chromatography; ultracentrifugation.
Figures




References
-
- Lönnerdal B. Bioactive proteins in human milk: mechanisms of action. J Pediatr. 2010;156:26–16. - PubMed
-
- Chatterton DEW, Nguyen DN, Bering SB. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int J Biochem Cell Biol. 2013;45:1730–1747. - PubMed
-
- Civardi E, Garofoli F, Mazzucchelli I. Enteral nutrition and infections: the role of human milk. Early Hum Dev. 2014;90:57–59. - PubMed
-
- Walker WA, Iyengar RS. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr Res. 2015;77:220–228. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

