Expression and function of ATP-dependent potassium channels in zebrafish islet β-cells
- PMID: 28386438
- PMCID: PMC5367309
- DOI: 10.1098/rsos.160808
Expression and function of ATP-dependent potassium channels in zebrafish islet β-cells
Abstract
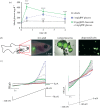
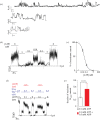
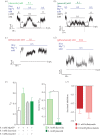
ATP-sensitive potassium channels (KATP channels) are critical nutrient sensors in many mammalian tissues. In the pancreas, KATP channels are essential for coupling glucose metabolism to insulin secretion. While orthologous genes for many components of metabolism-secretion coupling in mammals are present in lower vertebrates, their expression, functionality and ultimate impact on body glucose homeostasis are unclear. In this paper, we demonstrate that zebrafish islet β-cells express functional KATP channels of similar subunit composition, structure and metabolic sensitivity to their mammalian counterparts. We further show that pharmacological activation of native zebrafish KATP using diazoxide, a specific KATP channel opener, is sufficient to disturb glucose tolerance in adult zebrafish. That β-cell KATP channel expression and function are conserved between zebrafish and mammals illustrates the evolutionary conservation of islet metabolic sensing from fish to humans, and lends relevance to the use of zebrafish to model islet glucose sensing and diseases of membrane excitability such as neonatal diabetes.
Keywords: KATP; ion channels; metabolism; pancreas; zebrafish.
Figures

References
-
- Keane K, Newsholme P. 2014. Metabolic regulation of insulin secretion. Vitamins Horm. 95, 1–33. (doi:10.1016/B978-0-12-800174-5.00001-6) - DOI - PubMed
-
- Drews G, Krippeit-Drews P, Dufer M. 2010. Electrophysiology of islet cells. In The islets of langerhans (ed. Islam MS.), pp. 115–163. Dordrecht, The Netherlands: Springer. - PubMed
-
- Remedi MS, Nichols CG. 2009. Hyperinsulinism and diabetes: genetic dissection of beta cell metabolism-excitation coupling in mice. Cell Metab. 10, 442–453. (doi:10.1016/j.cmet.2009.10.011) - DOI - PMC - PubMed
-
- Koster JC, Permutt MA, Nichols CG. 2006. Diabetes and insulin secretion: the ATP-sensitive K+ channel (KATP) connection. Diabetes 54, 3065–3072. (doi:10.2337/diabetes.54.11.3065) - DOI - PubMed
-
- Gloyn AL, et al. 2004. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N. Engl. J. Med. 350, 1838–1849. (doi:10.1056/NEJMoa032922) - DOI - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

