Roles of tau protein in health and disease
- PMID: 28386764
- PMCID: PMC5390006
- DOI: 10.1007/s00401-017-1707-9
Roles of tau protein in health and disease
Abstract
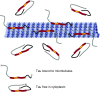
Tau is well established as a microtubule-associated protein in neurons. However, under pathological conditions, aberrant assembly of tau into insoluble aggregates is accompanied by synaptic dysfunction and neural cell death in a range of neurodegenerative disorders, collectively referred to as tauopathies. Recent advances in our understanding of the multiple functions and different locations of tau inside and outside neurons have revealed novel insights into its importance in a diverse range of molecular pathways including cell signalling, synaptic plasticity, and regulation of genomic stability. The present review describes the physiological and pathophysiological properties of tau and how these relate to its distribution and functions in neurons. We highlight the post-translational modifications of tau, which are pivotal in defining and modulating tau localisation and its roles in health and disease. We include discussion of other pathologically relevant changes in tau, including mutation and aggregation, and how these aspects impinge on the propensity of tau to propagate, and potentially drive neuronal loss, in diseased brain. Finally, we describe the cascade of pathological events that may be driven by tau dysfunction, including impaired axonal transport, alterations in synapse and mitochondrial function, activation of the unfolded protein response and defective protein degradation. It is important to fully understand the range of neuronal functions attributed to tau, since this will provide vital information on its involvement in the development and pathogenesis of disease. Such knowledge will enable determination of which critical molecular pathways should be targeted by potential therapeutic agents developed for the treatment of tauopathies.
Keywords: Alzheimer’s disease; Microtubule binding; Propagation; Synaptic dysfunction; Tau; Tauopathy.
Figures



References
-
- Abraha A, Ghoshal N, Gamblin TC, Cryns V, Berry RW, Kuret J, Binder LI. J Cell Sci. 2000;113:3737–3745. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

