Differential contribution of immune effector mechanisms to cortical demyelination in multiple sclerosis
- PMID: 28386765
- PMCID: PMC5486638
- DOI: 10.1007/s00401-017-1706-x
Differential contribution of immune effector mechanisms to cortical demyelination in multiple sclerosis
Abstract
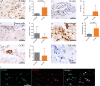
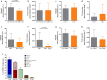
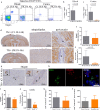
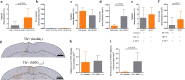
Cortical demyelination is a widely recognized hallmark of multiple sclerosis (MS) and correlate of disease progression and cognitive decline. The pathomechanisms initiating and driving gray matter damage are only incompletely understood. Here, we determined the infiltrating leukocyte subpopulations in 26 cortical demyelinated lesions of biopsied MS patients and assessed their contribution to cortical lesion formation in a newly developed mouse model. We find that conformation-specific anti-myelin antibodies contribute to cortical demyelination even in the absence of the classical complement pathway. T cells and natural killer cells are relevant for intracortical type 2 but dispensable for subpial type 3 lesions, whereas CCR2+ monocytes are required for both. Depleting CCR2+ monocytes in marmoset monkeys with experimental autoimmune encephalomyelitis using a novel humanized CCR2 targeting antibody translates into significantly less cortical demyelination and disease severity. We conclude that biologics depleting CCR2+ monocytes might be attractive candidates for preventing cortical lesion formation and ameliorating disease progression in MS.
Keywords: Cortical demyelination; Experimental autoimmune encephalomyelitis; Inflammatory monocytes; Progressive multiple sclerosis.
Conflict of interest statement
MM, MP and WB hold a patent (9068002) for the depletion of CCR2+ monocytes in MS.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

