Novel conformation-selective alpha-synuclein antibodies raised against different in vitro fibril forms show distinct patterns of Lewy pathology in Parkinson's disease
- PMID: 28386933
- PMCID: PMC5632188
- DOI: 10.1111/nan.12402
Novel conformation-selective alpha-synuclein antibodies raised against different in vitro fibril forms show distinct patterns of Lewy pathology in Parkinson's disease
Abstract
Aims: The aim of this study was to test the hypothesis that different conformations of misfolded α-synuclein (α-syn) are present in Parkinson's disease (PD) brain.
Methods: Using two previously characterized conformations of α-syn fibrils, we generated new conformation-selective α-syn monoclonal antibodies (mAbs). We then interrogated multiple brain regions in a well-characterized autopsy cohort of PD patients (n = 49) with these mAbs, Syn7015 and Syn9029.
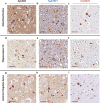
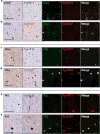
Results: Syn7015 detects Lewy bodies (LBs) and Lewy neurites (LNs) formed by pathological α-syn in all brain regions tested, and is particularly sensitive to LNs and small Lewy dots, inclusions believed to form early in the disease. Further, we observed colocalization between Syn7015 and an early marker of α-syn pathology formation, phospho-Ser129-α-syn, and a lack of extensive colocalization with markers of more mature pathology. In comparison, Syn9029 detects Lewy pathology in all regions examined, but indicates significantly fewer LNs than Syn7015. In addition, colocalization of Syn9029 with later markers of α-syn pathology maturation (ubiquitin and P62) suggests that the pathology detected by Syn9029 is older. Semiquantitative scoring of both LN and LB pathology in nine brain regions further established this trend, with Syn7015 LN scores consistently higher than Syn9029 LN scores.
Conclusions: Our data indicate that different conformations of α-syn pathology are present in PD brain and correspond to different stages of maturity for Lewy pathology. Regional analysis of Syn7015 and Syn9029 immunostaining also provides support for the Braak hypothesis that α-syn pathology advances through the brain.
Keywords: Lewy pathology maturation; Parkinson's disease; amyloid; conformation-selective antibodies.
© 2017 British Neuropathological Society.
Conflict of interest statement
All authors declare that they have no conflict of interest.
Figures





Comment in
-
Parkinson disease: Antibodies reveal age of Lewy pathology in PD.Nat Rev Neurol. 2017 Jun;13(6):318-319. doi: 10.1038/nrneurol.2017.62. Epub 2017 Apr 28. Nat Rev Neurol. 2017. PMID: 28452352 No abstract available.
References
-
- Forman MS, Trojanowski JQ, Lee VMY. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004;10:1055–63. - PubMed
-
- Braak H, Sandmann-Keil D, Gai W, Braak E. Extensive axonal Lewy neurites in Parkinson’s disease: a novel pathological feature revealed by alpha-synuclein immunocytochemistry. Neurosci Lett. 1999;265:67–9. - PubMed
-
- Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M. alpha-Synuclein in Lewy bodies. Nature. 1997;388:839–40. - PubMed
-
- Breydo L, Wu JW, Uversky VN. α-Synuclein misfolding and Parkinson’s disease. Biochim Biophys Acta. 2012;1822:261–85. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

