Calcium oxalate crystals and oxalate induce an epithelial-to-mesenchymal transition in the proximal tubular epithelial cells: Contribution to oxalate kidney injury
- PMID: 28387228
- PMCID: PMC5384284
- DOI: 10.1038/srep45740
Calcium oxalate crystals and oxalate induce an epithelial-to-mesenchymal transition in the proximal tubular epithelial cells: Contribution to oxalate kidney injury
Abstract
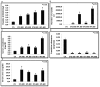
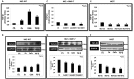
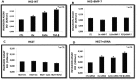
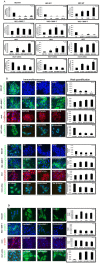
TGF-β1 is the main mediator of epithelial-to-mesenchymal transition (EMT). Hyperoxaluria induces crystalluria, interstitial fibrosis, and progressive renal failure. This study analyzed whether hyperoxaluria is associated with TGF-β1 production and kidney fibrosis in mice and if oxalate or calcium oxalate (CaOx) could induce EMT in proximal tubule cells (HK2) and therefore contribute to the fibrotic process. Hyperoxaluria was induced by adding hydroxyproline and ethylene glycol to the mice's drinking water for up to 60 days. Renal function and oxalate and urinary crystals were evaluated. Kidney collagen production and TGF-β1 expression were assessed. EMT was analyzed in vitro according to TGF-β1 production, phenotypic characterization, invasion, cell migration, gene and protein expression of epithelial and mesenchymal markers. Hyperoxaluric mice showed a decrease in renal function and an increase in CaOx crystals and Ox urinary excretion. The deposition of collagen in the renal interstitium was observed. HK2 cells stimulated with Ox and CaOx exhibited a decreased expression of epithelial as well as increased expression mesenchymal markers; these cells presented mesenchymal phenotypic changes, migration, invasiveness capability and TGF-β1 production, characterizing EMT. Treatment with BMP-7 or its overexpression in HK2 cells was effective at preventing it. This mechanism may contribute to the fibrosis observed in hyperoxaluria.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Miller N. L., Evan A. P. & Lingeman J. E. Pathogenesis of Renal Calculi. Urologic Clinics of North America 34, 295–313 (2007). - PubMed
-
- Zeisberg M. & Kalluri R. The role of epithelial-to-mesenchymal transition in renal fibrosis. Journal of Molecular Medicine 82, 175–181 (2004). - PubMed
-
- Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J. Am. Soc. Nephrol. 15, 1–12 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

