Targeting microbial biofilms using Ficin, a nonspecific plant protease
- PMID: 28387349
- PMCID: PMC5384253
- DOI: 10.1038/srep46068
Targeting microbial biofilms using Ficin, a nonspecific plant protease
Abstract
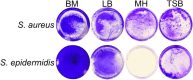
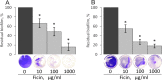
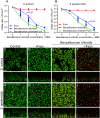
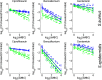
Biofilms, the communities of surface-attached bacteria embedded into extracellular matrix, are ubiquitous microbial consortia securing the effective resistance of constituent cells to environmental impacts and host immune responses. Biofilm-embedded bacteria are generally inaccessible for antimicrobials, therefore the disruption of biofilm matrix is the potent approach to eradicate microbial biofilms. We demonstrate here the destruction of Staphylococcus aureus and Staphylococcus epidermidis biofilms with Ficin, a nonspecific plant protease. The biofilm thickness decreased two-fold after 24 hours treatment with Ficin at 10 μg/ml and six-fold at 1000 μg/ml concentration. We confirmed the successful destruction of biofilm structures and the significant decrease of non-specific bacterial adhesion to the surfaces after Ficin treatment using confocal laser scanning and atomic force microscopy. Importantly, Ficin treatment enhanced the effects of antibiotics on biofilms-embedded cells via disruption of biofilm matrices. Pre-treatment with Ficin (1000 μg/ml) considerably reduced the concentrations of ciprofloxacin and bezalkonium chloride required to suppress the viable Staphylococci by 3 orders of magnitude. We also demonstrated that Ficin is not cytotoxic towards human breast adenocarcinoma cells (MCF7) and dog adipose derived stem cells. Overall, Ficin is a potent tool for staphylococcal biofilm treatment and fabrication of novel antimicrobial therapeutics for medical and veterinary applications.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

