Klotho preservation via histone deacetylase inhibition attenuates chronic kidney disease-associated bone injury in mice
- PMID: 28387374
- PMCID: PMC5384196
- DOI: 10.1038/srep46195
Klotho preservation via histone deacetylase inhibition attenuates chronic kidney disease-associated bone injury in mice
Abstract
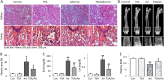
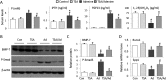
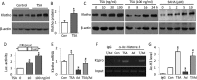
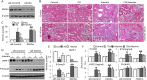
Bone loss and increased fracture are the devastating outcomes of chronic kidney disease-mineral and bone disorder (CKD-MBD) resulting from Klotho deficit-related mineral disturbance and hyperparathyroidism. Because Klotho down-regulation after renal injury is presumably affected by aberrant histone deacetylase (HDAC) activities, here we assess whether HDAC inhibition prevents Klotho loss and attenuates the CKD-associated bone complication in a mouse model of CKD-MBD. Mice fed adenine-containing diet developed the expected renal damage, a substantial Klotho loss and the deregulated key factors causally affecting bone remodeling, which were accompanied by a marked reduction of bone mineral density. Intriguingly, administration of a potent HDAC inhibitor trichostatin A (TSA) impressively alleviated the Klotho deficit and the observed alterations of serum, kidney and bone. TSA prevented Klotho loss by increasing the promoter-associated histone acetylation, therefore increasing Klotho transcription. More importantly the mice lacking Klotho by siRNA interference largely abolished the TSA protections against the serum and renal abnormalities, and the deranged bone micro-architectures. Thus, our study identified Klotho loss as a key event linking HDAC deregulation to the renal and bone injuries in CKD-MBD mice and demonstrated the therapeutic potentials of endogenous Klotho restoration by HDAC inhibition in treating CKD and the associated extrarenal complications.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Komaba H. [The expanding concept of CKD-MBD]. Clinical calcium 26, 1253–1260, doi: CliCa160912531260 (2016). - PubMed
-
- Iwashita Y., Iwashita Y., Ito T. & Shigematsu T. [Treatment for CKD-MBD(Chronic Kidney Disease-Mineral and Bone Disorder)]. Clinical calcium 26, 259–267, doi: CliCa1602259267 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

