Identification of human IgG1 variant with enhanced FcRn binding and without increased binding to rheumatoid factor autoantibody
- PMID: 28387635
- PMCID: PMC5524163
- DOI: 10.1080/19420862.2017.1314873
Identification of human IgG1 variant with enhanced FcRn binding and without increased binding to rheumatoid factor autoantibody
Abstract
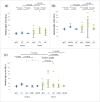
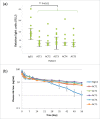
Various studies have demonstrated that Fc engineering to enhance neonatal Fc receptor (FcRn) binding is effective for elongating half-life or increasing cellular uptake of IgG. A previous study has shown that a N434H mutation to enhance FcRn binding resulted in increased binding to rheumatoid factor (RF) autoantibody, which is not desirable for therapeutic use in autoimmune disease. In this study, we first showed that all the existing Fc variants with enhanced FcRn binding also show increased RF binding, and then identified specific mutations that could be introduced to those Fc variants to reduce the RF binding. Furthermore, we generated novel Fc variants that do not increase RF binding and show half-lives of 45 d in cynomolgus monkey, which is longer than those of previously reported Fc variants. In addition, we generated novel Fc variants with antigen sweeping activity that do not increase RF binding. We expect that these novel Fc variants will be useful as antibody therapeutics against autoimmune diseases.
Keywords: Antibody therapeutics; CH2-CH3 interface; Fc engineering; FcRn; RF; pharmacokinetics; pre-existing ADA; rheumatoid factor; sweeping antibody.
Figures


References
-
- Kalden JR, Burkhardt H. Autoimmune disease: Treatment. Encyclopedia of Life Sciences 2009; https://doi.org/ 10.1002/9780470015902.a0001437.pub2 - DOI
-
- Rosman Z, Shoenfeld Y, Zandman-Goddard G. Biologic therapy for autoimmune diseases: An update. BMC Med 2013; 11:88; PMID: 23557513; https://doi.org/ 10.1186/1741-7015-11-88 - DOI - PMC - PubMed
-
- Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol 2010; 10:301-16; PMID: 20414204; https://doi.org/ 10.1038/nri2761 - DOI - PubMed
-
- Wang D, Li Y, Liu Y, Shi G. The use of biologic therapies in the treatment of rheumatoid arthritis. Curr Pharm Biotechnol 2014; 15:542-8; PMID: 25213363; https://doi.org/ 10.2174/138920101506140910150612 - DOI - PubMed
-
- Lycke J. Monoclonal antibody therapies for the treatment of relapsing-remitting multiple sclerosis: Differentiating mechanisms and clinical outcomes. Ther Adv Neurol Disord 2015; 8:274-93; PMID: 26600872; https://doi.org/ 10.1177/1756285615605429 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
