Advances in Skin Regeneration Using Tissue Engineering
- PMID: 28387714
- PMCID: PMC5412373
- DOI: 10.3390/ijms18040789
Advances in Skin Regeneration Using Tissue Engineering
Abstract
Tissue engineered skin substitutes for wound healing have evolved tremendously over the last couple of years. New advances have been made toward developing skin substitutes made up of artificial and natural materials. Engineered skin substitutes are developed from acellular materials or can be synthesized from autologous, allograft, xenogenic, or synthetic sources. Each of these engineered skin substitutes has their advantages and disadvantages. However, to this date, a complete functional skin substitute is not available, and research is continuing to develop a competent full thickness skin substitute product that can vascularize rapidly. There is also a need to redesign the currently available substitutes to make them user friendly, commercially affordable, and viable with longer shelf life. The present review focuses on providing an overview of advances in the field of tissue engineered skin substitute development, the availability of various types, and their application.
Keywords: skin regeneration; skin substitutes; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
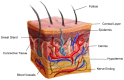
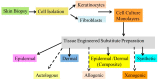

References
-
- Rheinwald J.G. Human epidermal keratinocyte cell culture and xenograft systems: Applications in the detection of potential chemical carcinogens and the study of epidermal transformation. Prog. Clin. Biol. Res. 1989;298:113–125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

