Recent Advances in Electrochemical Immunosensors
- PMID: 28387718
- PMCID: PMC5422067
- DOI: 10.3390/s17040794
Recent Advances in Electrochemical Immunosensors
Abstract
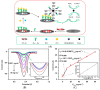
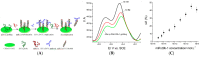
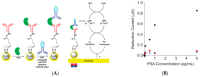
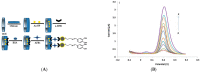
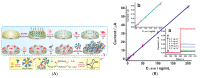
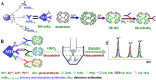
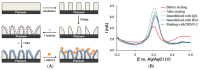
Immunosensors have experienced a very significant growth in recent years, driven by the need for fast, sensitive, portable and easy-to-use devices to detect biomarkers for clinical diagnosis or to monitor organic pollutants in natural or industrial environments. Advances in the field of signal amplification using enzymatic reactions, nanomaterials such as carbon nanotubes, graphene and graphene derivatives, metallic nanoparticles (gold, silver, various oxides or metal complexes), or magnetic beads show how it is possible to improve collection, binding or transduction performances and reach the requirements for realistic clinical diagnostic or environmental control. This review presents these most recent advances; it focuses first on classical electrode substrates, then moves to carbon-based nanostructured ones including carbon nanotubes, graphene and other carbon materials, metal or metal-oxide nanoparticles, magnetic nanoparticles, dendrimers and, to finish, explore the use of ionic liquids. Analytical performances are systematically covered and compared, depending on the detection principle, but also from a chronological perspective, from 2012 to 2016 and early 2017.
Keywords: ELISA; antibodies; carbon nanotubes; competitive immunosensor; dendrimers; electrochemical sensors; enzyme; graphene; hapten; immunosensors; ionic liquids; magnetic nanoparticles; nanoparticules; redox probe; sandwich-type immunosensors.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data and in the writing of the manuscript.
Figures
















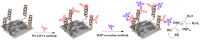



















References
-
- Ricci F., Adornetto G., Palleschi G. A review of experimental aspects of electrochemical immunosensors. Electrochim. Acta. 2012;84:74–83. doi: 10.1016/j.electacta.2012.06.033. - DOI
-
- Hasanzadeh M., Shadjou N., Omidinia E., Eskandani M., de la Guardia M. Mesoporous silica materials for use in electrochemical immunosensing. Trends Anal. Chem. 2012;45:93–106. doi: 10.1016/j.trac.2012.12.017. - DOI
-
- Arduini F., Micheli L., Moscone D., Palleschi G., Piermarini S., Ricci F., Volpe G. Electrochemical biosensors based on nanomodified screen-printed electrodes: Recent applications in clinical analysis. Trends Anal. Chem. 2016;79:114–126. doi: 10.1016/j.trac.2016.01.032. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

