Classical and Novel TSPO Ligands for the Mitochondrial TSPO Can Modulate Nuclear Gene Expression: Implications for Mitochondrial Retrograde Signaling
- PMID: 28387723
- PMCID: PMC5412370
- DOI: 10.3390/ijms18040786
Classical and Novel TSPO Ligands for the Mitochondrial TSPO Can Modulate Nuclear Gene Expression: Implications for Mitochondrial Retrograde Signaling
Abstract
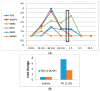
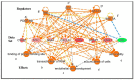
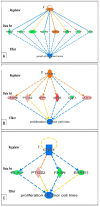
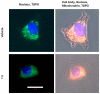
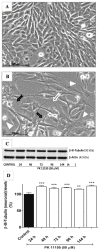
It is known that knockdown of the mitochondrial 18 kDa translocator protein (TSPO) as well as TSPO ligands modulate various functions, including functions related to cancer. To study the ability of TSPO to regulate gene expression regarding such functions, we applied microarray analysis of gene expression to U118MG glioblastoma cells. Within 15 min, the classical TSPO ligand PK 11195 induced changes in expression of immediate early genes and transcription factors. These changes also included gene products that are part of the canonical pathway serving to modulate general gene expression. These changes are in accord with real-time, reverse transcriptase (RT) PCR. At the time points of 15, 30, 45, and 60 min, as well as 3 and 24 h of PK 11195 exposure, the functions associated with the changes in gene expression in these glioblastoma cells covered well known TSPO functions. These functions included cell viability, proliferation, differentiation, adhesion, migration, tumorigenesis, and angiogenesis. This was corroborated microscopically for cell migration, cell accumulation, adhesion, and neuronal differentiation. Changes in gene expression at 24 h of PK 11195 exposure were related to downregulation of tumorigenesis and upregulation of programmed cell death. In the vehicle treated as well as PK 11195 exposed cell cultures, our triple labeling showed intense TSPO labeling in the mitochondria but no TSPO signal in the cell nuclei. Thus, mitochondrial TSPO appears to be part of the mitochondria-to-nucleus signaling pathway for modulation of nuclear gene expression. The novel TSPO ligand 2-Cl-MGV-1 appeared to be very specific regarding modulation of gene expression of immediate early genes and transcription factors.
Keywords: 2-Cl-MGV-1; PK 11195; TSPO ligand; cell nucleus; microscopy; mitochondria; mitochondrial 18 kDa translocator protein (TSPO); modulation of nuclear gene expression; retrograde mitochondrial-nuclear signaling pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Gavish M., Bachman I., Shoukrun R., Katz Y., Veenman L., Weisinger G., Weizman A. Enigma of the peripheral benzodiazepine receptor. Pharmacol. Rev. 1999;51:629–650. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

