Phase 1 Dose-Escalation Study of Pegylated Arginine Deiminase, Cisplatin, and Pemetrexed in Patients With Argininosuccinate Synthetase 1-Deficient Thoracic Cancers
- PMID: 28388291
- PMCID: PMC6141244
- DOI: 10.1200/JCO.2016.71.3230
Phase 1 Dose-Escalation Study of Pegylated Arginine Deiminase, Cisplatin, and Pemetrexed in Patients With Argininosuccinate Synthetase 1-Deficient Thoracic Cancers
Abstract
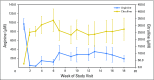
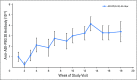
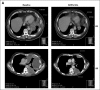
Purpose Pegylated arginine deiminase (ADI-PEG 20) depletes essential amino acid levels in argininosuccinate synthetase 1 (ASS1) -negative tumors by converting arginine to citrulline and ammonia. The main aim of this study was to determine the recommended dose, safety, and tolerability of ADI-PEG 20, cisplatin, and pemetrexed in patients with ASS1-deficient malignant pleural mesothelioma (MPM) or non-small-cell lung cancer (NSCLC). Patients and Methods Using a 3 + 3 + 3 dose-escalation study, nine chemotherapy-naïve patients (five MPM, four NSCLC) received weekly ADI-PEG 20 doses of 18 mg/m2, 27 mg/m2, or 36 mg/m2, together with pemetrexed 500 mg/m2 and cisplatin 75 mg/m2 which were given every three weeks (maximum of six cycles). Patients achieving stable disease or better could continue ADI-PEG 20 monotherapy until disease progression or withdrawal. Adverse events were assessed by Common Terminology Criteria for Adverse Events version 4.03, and pharmacodynamics and immunogenicity were also evaluated. Tumor response was assessed by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 for NSCLC and by modified RECIST criteria for MPM. Results No dose-limiting toxicities were reported; nine of 38 reported adverse events (all grade 1 or 2) were related to ADI-PEG 20. Circulating arginine concentrations declined rapidly, and citrulline levels increased; both changes persisted at 18 weeks. Partial responses were observed in seven of nine patients (78%), including three with either sarcomatoid or biphasic MPM. Conclusion Target engagement with depletion of arginine was maintained throughout treatment with no dose-limiting toxicities. In this biomarker-selected group of patients with ASS1-deficient cancers, clinical activity was observed in patients with poor-prognosis tumors. Therefore, we recommend a dose for future studies of weekly ADI-PEG 20 36 mg/m2 plus three-weekly cisplatin 75 mg/m2 and pemetrexed 500 mg/m2.
Figures


Comment in
-
Combination of Arginine Depletion and Chemotherapy in Thoracic Malignancies.J Clin Oncol. 2017 Jun 1;35(16):1758-1759. doi: 10.1200/JCO.2017.72.7305. Epub 2017 Apr 14. J Clin Oncol. 2017. PMID: 28410012 No abstract available.
References
-
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. : Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21:2636-2644, 2003 - PubMed
-
- Scagliotti GV, Parikh P, von Pawel J, et al. : Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26:3543-3551, 2008 - PubMed
-
- Delage B, Fennell DA, Nicholson L, et al. : Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int J Cancer 126:2762-2772, 2010 - PubMed
-
- Wheatley DN, Philip R, Campbell E: Arginine deprivation and tumour cell death: Arginase and its inhibition. Mol Cell Biochem 244:177-185, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

