Trade-off between Transcriptome Plasticity and Genome Evolution in Cephalopods
- PMID: 28388405
- PMCID: PMC5499236
- DOI: 10.1016/j.cell.2017.03.025
Trade-off between Transcriptome Plasticity and Genome Evolution in Cephalopods
Abstract
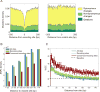
RNA editing, a post-transcriptional process, allows the diversification of proteomes beyond the genomic blueprint; however it is infrequently used among animals for this purpose. Recent reports suggesting increased levels of RNA editing in squids thus raise the question of the nature and effects of these events. We here show that RNA editing is particularly common in behaviorally sophisticated coleoid cephalopods, with tens of thousands of evolutionarily conserved sites. Editing is enriched in the nervous system, affecting molecules pertinent for excitability and neuronal morphology. The genomic sequence flanking editing sites is highly conserved, suggesting that the process confers a selective advantage. Due to the large number of sites, the surrounding conservation greatly reduces the number of mutations and genomic polymorphisms in protein-coding regions. This trade-off between genome evolution and transcriptome plasticity highlights the importance of RNA recoding as a strategy for diversifying proteins, particularly those associated with neural function. PAPERCLIP.
Keywords: ADAR; Epitranscriptome; RNA editing; RNA modifications; cephalopods; genome evolution; neural plasticity; proteome diversity.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

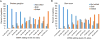
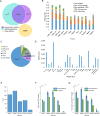
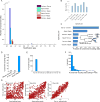
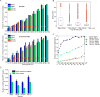

Comment in
-
Evolutionary genetics: Fantastic beasts - cephalopod RNA recoding.Nat Rev Genet. 2017 Jun;18(6):329. doi: 10.1038/nrg.2017.31. Epub 2017 Apr 19. Nat Rev Genet. 2017. PMID: 28420879 No abstract available.
-
Shaping and Reshaping Transcriptome Plasticity during Evolution.Trends Biochem Sci. 2017 Sep;42(9):682-684. doi: 10.1016/j.tibs.2017.06.009. Epub 2017 Jul 14. Trends Biochem Sci. 2017. PMID: 28716332
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

