Aneuploidy Causes Non-genetic Individuality
- PMID: 28388408
- PMCID: PMC5441241
- DOI: 10.1016/j.cell.2017.03.021
Aneuploidy Causes Non-genetic Individuality
Abstract
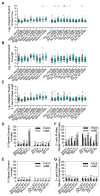
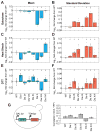
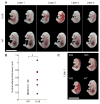
Phenotypic variability is a hallmark of diseases involving chromosome gains and losses, such as Down syndrome and cancer. Allelic variances have been thought to be the sole cause of this heterogeneity. Here, we systematically examine the consequences of gaining and losing single or multiple chromosomes to show that the aneuploid state causes non-genetic phenotypic variability. Yeast cell populations harboring the same defined aneuploidy exhibit heterogeneity in cell-cycle progression and response to environmental perturbations. Variability increases with degree of aneuploidy and is partly due to gene copy number imbalances, suggesting that subtle changes in gene expression impact the robustness of biological networks and cause alternate behaviors when they occur across many genes. As inbred trisomic mice also exhibit variable phenotypes, we further propose that non-genetic individuality is a universal characteristic of the aneuploid state that may contribute to variability in presentation and treatment responses of diseases caused by aneuploidy.
Keywords: Down syndrome; aneuploidy; biological noise; cancer; cell-to-cell variability; gene dosage effects; non-genetic heterogeneity.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




References
-
- Acar M, Mettetal JT, van Oudenaarden A. Stochastic switching as a survival strategy in fluctuating environments. Nat Genet. 2008;40:471–475. - PubMed
-
- Alvaro D, Sunjevaric I, Reid RJD, Lisby M, Stillman DJ, Rothstein R. Systematic hybrid LOH: a new method to reduce false positives and negatives during screening of yeast gene deletion libraries. Yeast Chichester Engl. 2006;23:1097–1106. - PubMed
-
- Arias AM, Hayward P. Filtering transcriptional noise during development: concepts and mechanisms. Nat Rev Genet. 2006;7:34–44. - PubMed
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

