ESCRT-III Acts Downstream of MLKL to Regulate Necroptotic Cell Death and Its Consequences
- PMID: 28388412
- PMCID: PMC5443414
- DOI: 10.1016/j.cell.2017.03.020
ESCRT-III Acts Downstream of MLKL to Regulate Necroptotic Cell Death and Its Consequences
Abstract
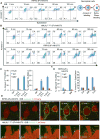
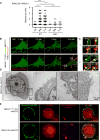
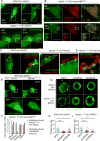
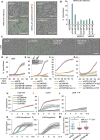
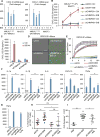
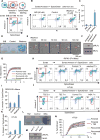
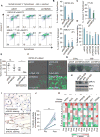
The activation of mixed lineage kinase-like (MLKL) by receptor-interacting protein kinase-3 (RIPK3) results in plasma membrane (PM) disruption and a form of regulated necrosis, called necroptosis. Here, we show that, during necroptosis, MLKL-dependent calcium (Ca2+) influx and phosphatidylserine (PS) exposure on the outer leaflet of the plasma membrane preceded loss of PM integrity. Activation of MLKL results in the generation of broken, PM "bubbles" with exposed PS that are released from the surface of the otherwise intact cell. The ESCRT-III machinery is required for formation of these bubbles and acts to sustain survival of the cell when MLKL activation is limited or reversed. Under conditions of necroptotic cell death, ESCRT-III controls the duration of plasma membrane integrity. As a consequence of the action of ESCRT-III, cells undergoing necroptosis can express chemokines and other regulatory molecules and promote antigenic cross-priming of CD8+ T cells.
Keywords: ESCRT-III; MLKL; annexin-V; necroptosis; phosphatidylserine; plasma membrane repair.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
Comment in
-
ESCRTing Necroptosis.Cell. 2017 Apr 6;169(2):186-187. doi: 10.1016/j.cell.2017.03.030. Cell. 2017. PMID: 28388403
-
Cell death: ESCRTing dying cells back to life.Nat Rev Mol Cell Biol. 2017 Jun;18(6):342-343. doi: 10.1038/nrm.2017.46. Epub 2017 Apr 21. Nat Rev Mol Cell Biol. 2017. PMID: 28429789 No abstract available.
-
Necroptosis: (Last) Message in a Bubble.Immunity. 2017 Jul 18;47(1):1-3. doi: 10.1016/j.immuni.2017.07.002. Immunity. 2017. PMID: 28723543
References
-
- Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. - PubMed
-
- Carlton J. The ESCRT machinery: a cellular apparatus for sorting and scission. Biochem Soc Trans. 2010;38:1397–1412. - PubMed
-
- Christ L, Raiborg C, Wenzel EM, Campsteijn C, Stenmark H. Cellular Functions and Molecular Mechanisms of the ESCRT Membrane-Scission Machinery. Trends Biochem Sci. 2017;42:42–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

