Involvement of heparanase in the pathogenesis of acute kidney injury: nephroprotective effect of PG545
- PMID: 28388547
- PMCID: PMC5470960
- DOI: 10.18632/oncotarget.16573
Involvement of heparanase in the pathogenesis of acute kidney injury: nephroprotective effect of PG545
Abstract
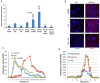
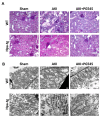
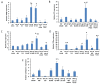
Despite the high prevalence of acute kidney injury (AKI) and its association with increased morbidity and mortality, therapeutic approaches for AKI are disappointing. This is largely attributed to poor understanding of the pathogenesis of AKI. Heparanase, an endoglycosidase that cleaves heparan sulfate, is involved in extracellular matrix turnover, inflammation, kidney dysfunction, diabetes, fibrosis, angiogenesis and cancer progression. The current study examined the involvement of heparanase in the pathogenesis of ischemic reperfusion (I/R) AKI in a mouse model and the protective effect of PG545, a potent heparanase inhibitor. I/R induced tubular damage and elevation in serum creatinine and blood urea nitrogen to a higher extent in heparanase over-expressing transgenic mice vs. wild type mice. Moreover, TGF-β, vimentin, fibronectin and α-smooth muscle actin, biomarkers of fibrosis, and TNFα, IL6 and endothelin-1, biomarkers of inflammation, were upregulated in I/R induced AKI, primarily in heparanase transgenic mice, suggesting an adverse role of heparanase in the pathogenesis of AKI. Remarkably, pretreatment of mice with PG545 abolished kidney dysfunction and the up-regulation of heparanase, pro-inflammatory (i.e., IL-6) and pro-fibrotic (i.e., TGF-β) genes induced by I/R. The present study provides new insights into the involvement of heparanase in the pathogenesis of ischemic AKI.Our results demonstrate that heparanase plays a deleterious role in the development of renal injury and kidney dysfunction,attesting heparanase inhibition as a promising therapeutic approach for AKI.
Keywords: PG545; acute kidney injury; heparanase; inflammation; ischemia.
Conflict of interest statement
Edward Hammond is employed by Zucero Therapeutics, Darra, Queensland, Australia.
Figures


References
-
- Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365:417–430. - PubMed
-
- Sheridan AM, Bonventre JV. Pathophysiology of ischemic acute renal failure. Contrib Nephrol. 2001;132:7–21. - PubMed
-
- Agarwal A, Nick HS. Renal response to tissue injury: lessons from heme oxygenase-1 GeneAblation and expression. J Am Soc Nephrol. 2000;11:965–973. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

