Synthesis, bioactivity, 3D-QSAR studies of novel dibenzofuran derivatives as PTP-MEG2 inhibitors
- PMID: 28388567
- PMCID: PMC5503546
- DOI: 10.18632/oncotarget.16595
Synthesis, bioactivity, 3D-QSAR studies of novel dibenzofuran derivatives as PTP-MEG2 inhibitors
Abstract
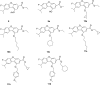
PTP-MEG2 plays a critical role in the diverse cell signalling processes, so targeting PTP-MEG2 is a promising strategy for various human diseases treatments. In this study, a series of novel dibenzofuran derivatives was synthesized and assayed for their PTP-MEG2 inhibitory activities. 10a with highest inhibitory activity (320 nM) exhibited significant selectivity for PTP-MEG2 over its close homolog SHP2, CDC25 (IC50 > 50 μM). By means of the powerful ''HipHop'' technique, a 3D-QSAR study was carried out to explore structure activity relationship of these molecules. The generated pharmacophore model revealed that the one RA, three Hyd, and two HBA features play an important role in binding to the active site of the target protein-PTP-MEG2. Docking simulation study indicated that 10a achieved its potency and specificity for PTP-MEG2 by targeting unique nearby peripheral binding pockets and the active site. The absorption, distribution, metabolism and excretion (ADME) predictions showed that the 11 compounds hold high potential to be novel lead compounds for targeting PTP-MEG2. Our findings here can provide a new strategy or useful insights for designing the effective PTP-MEG2 inhibitors.
Keywords: 3D-QSAR; PTP-MEG2; dibenzofuran; docking; synthesis.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



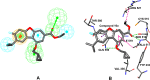
References
-
- Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833–846. - PubMed
-
- Zhang ZY. Protein tyrosine phosphatases: prospects for therapeutics. Curr Opin Chem Biol. 2001;5:416–423. - PubMed
-
- Julien SG, Dube N, Hardy S, Tremblay ML. Inside the human cancer tyrosine phosphatome. Nat Rev Cancer. 2011;11:35–49. - PubMed
-
- Yi T, Lindner D. The role and target potential of protein tyrosine phosphatases in cancer. Curr Oncol Rep. 2008;10:114–121. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

