Viral and serological kinetics in Zika virus-infected patients in South Korea
- PMID: 28388922
- PMCID: PMC5383943
- DOI: 10.1186/s12985-017-0740-6
Viral and serological kinetics in Zika virus-infected patients in South Korea
Abstract
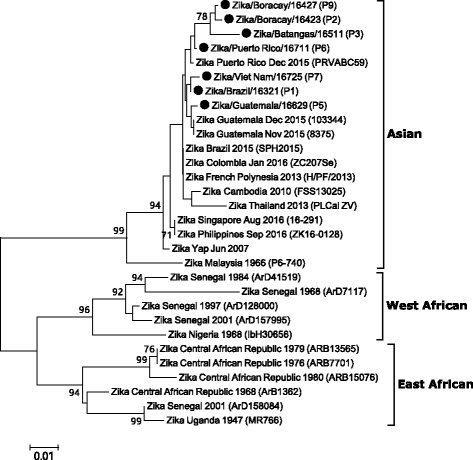
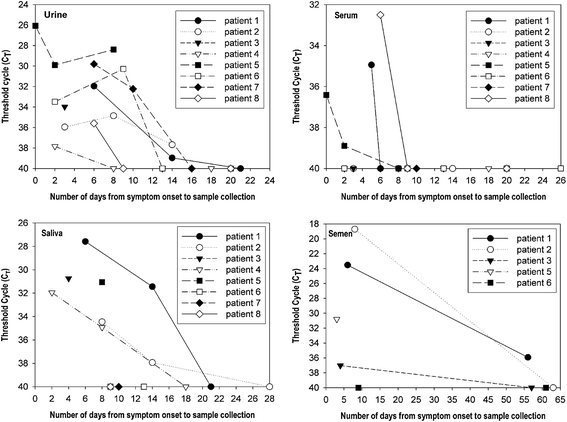
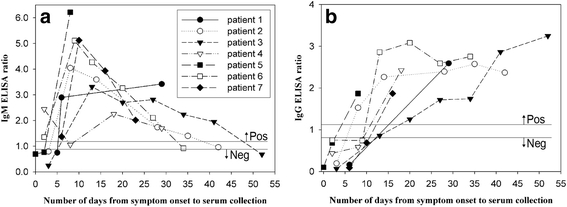
Zika virus is a mosquito-borne flavivirus that causes clinical symptoms similar to those observed in dengue and chikungunya virus infections. The Korea Centers for Disease Control and Prevention initiated laboratory testing using a real-time reverse transcription-polymerase chain reaction in January 2016. More than 1,000 suspected cases of infection were tested and nine were confirmed as imported cases of Zika virus infection from January to July 2016. The travel destinations of the infected individuals were Brazil, Philippines, Viet Nam, Guatemala, Puerto Rico, and the Dominican Republic. Phylogenetic analysis based on the partial envelope gene indicated that the viruses belonged to the Asian genotype circulating in South America. We further investigated the duration for which the viral RNA and virus-specific antibodies were detectable after the symptom onset. After the day of symptom onset, Zika virus was detectable until 6 days in serum, 14 days in urine and saliva, and 58 days in semen. Immunoglobulin M against Zika virus was detected as early as 2 days after the symptom onset and was maintained at these levels until 41 days, whereas Immunoglobulin G was detectable from 8 days after the symptom onset and was maintained until 52 days. These findings would help diagnostic laboratories improve their testing programs for Zika virus infection.
Keywords: Chikungunya virus; Dengue virus; Enzyme-Linked Immunosorbent Assay; Phylogenetic analysis; Reverse transcription-polymerase chain reaction; Zika virus.
Figures
References
-
- Huzly D, Hanselmann I, Schmidt-Chanasit J, Panning M. High specificity of a novel Zika virus ELISA in European patients after exposure to different flaviviruses. Euro Surveill. 2016;21(16). doi:10.2807/1560-7917.ES.2016.21.16.30203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

